

|

|

Volume
7: No. 1, January 2010
ORIGINAL RESEARCH
Health Behaviors and Quality of Life of Cancer Survivors in Massachusetts, 2006: Data
Use for Comprehensive Cancer Control
Temeika L. Fairley, PhD; Helen Hawk, PhD; Snaltze Pierre, MPH
Suggested citation for this article: Fairley TL, Hawk H, Pierre S. Health behaviors and quality of life of cancer survivors in Massachusetts, 2006: data use for comprehensive cancer control. Prev Chronic Dis 2010;7(1):A09.
http://www.cdc.gov/pcd/issues/2010/
jan/09_0062.htm. Accessed [date].
PEER REVIEWED
Abstract
Introduction
Nearly 12 million cancer survivors are living in the United States. Few state-based studies have examined the health status and health-related quality of life (HRQOL) of this growing population. The objective of this study was to use Massachusetts Behavioral Risk Factor Surveillance System (BRFSS) data to describe cancer survivors’ demographics, health behaviors, quality of life, use of preventive care services, and influenza
vaccination rates.
Methods
The demographic characteristics of cancer survivors and respondents
without cancer were estimated on the basis of responses to questions in the 2006 Massachusetts BRFSS.
We used multivariate logistic regression to compare health behaviors, comorbidities, quality of life, and cancer screening and influenza vaccination rates for cancer survivors compared
with respondents who did not have cancer.
Results
Cancer survivors and respondents who did not have cancer had similar rates
of health behavioral risk factors including smoking, obesity, and physical activity. Rates of chronic disease (eg, heart disease, asthma) and disability were higher among cancer survivors. Cancer survivors reported higher
rates of influenza vaccination
and breast, colorectal, and cervical cancer screening than did respondents who did not have cancer. Survivors’ self-reported health status and HRQOL
(physical and mental health) improved as length of survivorship increased.
Conclusion
This state-based survey allowed Massachusetts to assess health-related issues for resident cancer survivors. These findings will help state-based public health planners develop interventions to address the long-term physical and psychosocial
consequences of cancer diagnosis and treatment.
Back to top
Introduction
Nearly 12 million cancer survivors are living in the United States (1). Approximately 66% of cancer patients are expected to live at least 5 years after diagnosis (2). As use of cancer screening tests increases, cancer treatments improve, and the US population ages, we can expect the number of cancer survivors to increase (3). Concerns about the long-term physical, psychological, and economic effects of cancer treatment on cancer survivors and their families are being recognized and
addressed by public, private, and nonprofit organizations (4). Increased recognition of the seriousness of these issues has contributed to the development of
responsive public health strategies such as the publication of A National Action Plan for Cancer Survivorship (5) by the Centers for Disease Control and Prevention (CDC) and
From Cancer Patient to Cancer Survivor: Lost in Transition (6) by the Institute of Medicine.
CDC’s National Comprehensive Cancer Control Program (NCCCP) funds states, tribes/tribal organizations, and selected US territories and
associated Pacific Island jurisdictions to develop and implement
local comprehensive cancer control plans (7). Most cancer plans include specific goals and objectives about survivorship (4). NCCCP programs need population-based data sources to assess the effectiveness of activities related to survivorship. Population-based data will
allow state-specific analyses of the health behaviors of cancer survivors. To
meet this need, CDC’s Division of Cancer Prevention and Control is sponsoring cancer survivorship questions on the 2009 Behavioral Risk Factor Surveillance System (BRFSS) survey.
Data from the 2006 Massachusetts BRFSS survey are used in the analysis presented
in this article to describe the demographic characteristics, health status, health-related quality of life (HRQOL), and health behaviors of cancer survivors and
respondents without cancer in Massachusetts.
Back to top
Methods
In the 2006 Massachusetts BRFSS, questions on cancer survivorship were asked of respondents aged 18 years or older. The initial question was “Have you ever been diagnosed with cancer?” and respondents could
answer yes, no, or “don’t know/not sure,” or refuse to answer. If they answered yes, respondents were then asked, “What type of cancer were you diagnosed as having?” and were provided a list of choices (lung, colorectal, prostate, breast, cervical, ovarian or
uterine, pancreatic, stomach or esophageal, liver/bile duct, urinary/bladder, non-Hodgkin lymphoma, leukemia, thyroid, oral cavity/pharynx, melanoma, or other [specify]). Respondents
could select up to 3 cancers from this list. Respondents were also asked, “In what month and year were you last diagnosed with cancer?”
We created a variable indicating cancer prevalence from the questions on cancer
that had been added by the state and from the core module question on prostate cancer. To estimate the prevalence among adults of
a history of cancer, we calculated the weighted percentage of all types of cancers together and for main cancer types.
Cancer survivors were compared to respondents without cancer on the following demographic characteristics: age, race/ethnicity (categorized as white, non-Hispanic; black, non-Hispanic; and all other), sex, employment status (collapsed into employed for wages, out of work/unable to work, other, and retired), marital status (collapsed into currently married/living together
and all other), and education level (collapsed into less than high school graduate and high school graduate
or more).
We selected the following indicators of health behavior, HRQOL, and health status to assess cancer survivors and
respondents without cancer: self-reported health status, disability status, leisure-time physical activity, smoking status, alcohol consumption, receipt of influenza vaccine in the past 12 months, and being up to date with age-appropriate cancer screenings.
We collapsed self-reported health status to 2 levels
(excellent, very good, and good; and fair and poor). HRQOL was measured by using the CDC
healthy days measures (mean physically unhealthy days in the past 30 days and mean mentally unhealthy days in the past 30 days) (8). Disability status was grouped as
having disability more than 1 year and other. Leisure-time physical activity or exercise during the past month (other than the respondent’s regular job) and binge drinking (men having 5 or more drinks on 1 occasion,
women having 4 or more drinks on 1 occasion) were grouped as yes and no. Smoking status was categorized as current (smoked at least 100 cigarettes in their lifetime and now smoke some days or every day), former (smoked at least 100 cigarettes in their lifetime and currently do not smoke), and never (have not smoked at least 100 cigarettes in their lifetime). Responses to the question about having received influenza vaccine in the past 12 months were grouped as yes
and no. Cancer screening
was assessed, including mammography in the past 2 years for women aged 40 years or older, Pap smears in the past 3 years
for women aged 18 years or older, colorectal cancer screening among men and women aged 50 years or older (within the past 5 years for endoscopy) and prostate cancer screening within the past 2 years among men aged 50 years or older (9). We assessed changes in self-reported health status and HRQOL by time since diagnosis. The length of cancer survivorship was
estimated as time since diagnosis to the date of completing the survey.
Data were weighted by using SAS version 9.1.3 (SAS Institute, Inc, Cary, North Carolina) to account for the complex sampling design.
We used multivariate logistic regression models controlling for sex and age to assess the association between cancer and variables of interest (obesity, tobacco use, physical inactivity, screening use, vaccination use). Cancer survivors were compared
to
respondents without cancer as a reference group. Significance was set at P < .05.
Back to top
Results
Overall, 8,091 people completed the Massachusetts BRFSS in 2006. Of these respondents, nearly 10% (n = 780)
reported having ever been diagnosed with cancer. We excluded respondents with unknown age at the time interview or unknown date of diagnosis, respondents who reported “don’t know/not sure” or refused to answer the question, respondents with nonmelanoma
skin cancers, and those with multiple cancers. The resulting sample included 231 men and
485 women. More than half of the cancer survivors reported being diagnosed 6 or more years ago (Table 1).
Compared with respondents
without cancer, more cancer survivors were older, female, and non-Hispanic white.
Fewer survivors were employed for wages or insured by private insurance companies.
We found no significant differences by education
level or marital status (Table 1).
Breast, cervical/ovarian/uterine, colorectal, and thyroid cancers and melanoma were the most frequently reported cancers among women
(Table 2). Among men,
prostate, colorectal, and bladder cancers; melanoma; and non-Hodgkin lymphoma were
the most frequently reported. Cancer sites were reported as “unknown” by 9% of men and 3% of women
who reported they had a history of cancer.
In the multivariate regression analysis, cancer survivors had similar
behavioral risks, such as for smoking, drinking, and being obese, compared
with respondents
without cancer,
but were less engaged in leisure-time physical activity (Table 3).
Rates of heart disease, asthma, and disability were higher in survivors than
in respondents without cancer. Cancer survivors were also significantly more likely to receive age-appropriate cancer screenings (except for prostate cancer screening) and influenza vaccination than
respondents without cancer.
Survivors’ self-reported health status and HRQOL (physical and mental health) improved significantly as length of survivorship increased (Figure). For example, the odds ratio for life satisfaction increased from 0.7 at 5 years or
fewer since diagnosis to 1.3 at more than 10 years since diagnosis.
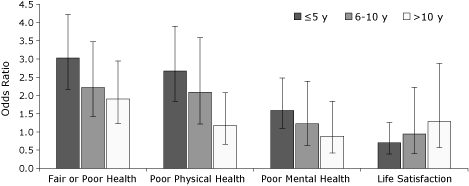
Figure. Health status and quality of life
among cancer survivors, by the
time since diagnosis, Massachusetts Behavioral Risk Factor Surveillance System,
2006. The reference group is respondents with no cancer diagnosed. Rates are
adjusted for age and sex. I-bars represent 95% confidence intervals. [A
tabular version of this figure is also available.]
Back to top
Discussion
Historically, comprehensive cancer control programs have relied on cancer incidence, mortality, and local survey data to describe
cancer in relation to cancer survivorship. The use of self-reported cancer prevalence data for cancer control at the state level is
rare because few programs have the capacity to collect these data. We found that
most cancer survivors were aged 55 years or older, regardless of sex. This pattern
was similar to that documented in the Massachusetts Cancer Registry (MCR) (Helen Hawk,
PhD, written communication, December 18, 2008). The distribution of self-reported cancers was also similar
to that documented in
the MCR (Helen Hawk, PhD, written communication, December 18, 2008). However, we observed variation in rankings of these cancers between these 2 systems.
Lung cancers were more frequently documented by the MCR than the BRFSS. Lung cancer patients may be institutionalized or too sick to participate in the BRFSS
telephone interviews. Their absence from survey data should be investigated through studies
of data from, for example, caregivers and hospitals.
Time since diagnosis affected self-reported health status and quality of life among cancer survivors. These findings are similar to
those of a previous study that assessed variation in HRQOL by time since diagnosis (10).
However, they differ from findings from several other studies (11-13) that described
health-related behaviors, HRQOL, and access to care among cancer survivors,
indicating that cancer survivors have a lower quality of life than
respondents without cancer.
Cancer survivors are also at increased risk of developing second cancers
because of risk factors that led to the first cancer or as a consequence of therapy (14). These risk factors have also been linked to treatment complications, reduced quality of life, and
mortality among cancer survivors (3,6,8,15). Since smoking cessation and increased exercise are associated with lower levels of cancer recurrence (8,15), appropriate activities
aimed at improving or modifying these health behaviors may improve the health of Massachusetts cancer survivors.
Cancer survivors may also have increased risk for chronic conditions such as
heart disease (3), diabetes (16), obesity-related asthma (17), and disability
(18). Little is known
about the effect of comorbid health conditions on diagnosis, treatment, subsequent health, or quality of life of cancer survivors; thus, further investigation into these relationships is warranted.
Cancer survivors in Massachusetts were more likely than respondents without cancer to receive age-appropriate screening for colorectal and cervical cancers,
a finding similar to one in a previous
study (19). However, the respondents did not differ significantly in receipt of screening for prostate and breast
cancers, which differs from the findings in a study
reporting that survivors were more likely to receive breast and prostate cancer screening than other respondents (19). Although screening guidelines recommend that young survivors receive screening at earlier ages (20),
the small sample size prevented us from examining screening use in this population. Future analyses, which will include multiple years of data, may allow us to assess screening behavior in
younger respondents.
Influenza vaccination is recommended for people with chronic diseases (21).
Cancer survivors are at increased risk of developing complications from influenza
(22). Therefore we examined vaccination use among Massachusetts survivors. We found that cancer survivors were significantly more likely than
respondents without cancer to report receipt of the influenza vaccine. Although we did not assess the
effect of age on vaccine use, prior studies noted that even in age-appropriate adults (23) only 59.2% of
cancer survivors reported receiving an influenza vaccination. These rates may be appropriate, however, depending on the time since diagnosis and whether cancer patients are being actively treated
for cancer (23).
Our findings are subject to several limitations. First, the survey may not be
representative of people who do not have a land-line telephone, which is
required for participation in the BRFSS survey (24). Second, BRFSS data are
self-reported and subject to recall bias, which could lead to inaccurate
estimates of cancer prevalence (25). Third, because our findings are limited to
noninstitutionalized US citizens, cancer survivors who may have advanced disease
and are living in nursing homes, long-term–care facilities, or hospice are not
included in our study. Fourth, because this survey does not collect information
from people younger than 18 years; thus, we are unable to describe the health
behaviors of this population. Fifth, low cooperation for the Massachusetts BRFSS
survey may also limit the generalizability of our study findings to all cancer
survivors living in Massachusetts. Although studies have concluded that the
national survey findings are reliable and valid (26), the reliability and
validity of state-level data have not been directly assessed. To accurately do
so, state-level BRFSS prevalence estimates must be compared with prevalence estimates from state cancer registries.
Sixth, we also lacked information about cancer stage at diagnosis and whether
the cancer diagnosis led to the development of other chronic conditions (eg,
heart disease, diabetes, asthma) or vice versa. Also, the number and intensity
of HRQOL issues vary with the type of cancer (27). Finally, the experience of cancer survivors in Massachusetts may differ from
that of others in the United States
because more than 95% of Massachusetts residents have health insurance (28). Increased access to health care as a result of health care reform initiatives may
affect the health behaviors, health status, and overall survivorship of people with cancer.
Studies
are needed to assess the effect of increased health care access on the health behaviors of cancer survivors.
State-level population-based data on the health and care of cancer survivors may be used by cancer control programs to tailor programs that meet the needs of cancer survivors. For example, the Massachusetts Comprehensive Cancer Prevention and Control Program (MCCPCP) and the Massachusetts Comprehensive Cancer Control
Coalition’s Survivorship Workgroup used their BRFSS data to help address potential challenges in the provision of health care and preventive services for cancer survivors (eg, treatment of chronic disease, risk factor education).
The MCCPCP has continued to support the collection of BRFSS data for cancer survivors.
The additional data may be used to identify the needs of Massachusetts cancer survivors in certain subpopulations (eg, racial/ethnic minority groups)
or with certain cancer types (eg, breast, colorectal, melanoma). Such
information will help us to develop interventions to improve the quality of care and quality of life
of cancer survivors.
Back to top
Author Information
Corresponding Author: Temeika L. Fairley, PhD, Division of Cancer Prevention and Control, Centers for Disease Control and Prevention, 4770 Buford Hwy, Mailstop K-57, Atlanta, GA 30341. Telephone: 770-488-4518. E-mail:
TFairley@cdc.gov.
Author Affiliations: Helen Hawk, Snaltze Pierre, Massachusetts Department of Public Health, Boston, Massachusetts.
Back to top
References
- Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al,
editors. SEER cancer statistics review, 1975-2006. Bethesda (MD): National
Cancer Institute, 2009.
- Cancer facts and figures. Atlanta (GA): American Cancer Society, 2008.
- Hewitt M, Rowland JH, Yancik R.
Cancer survivors in the United States: age, health and disability. J Gerontol A Biol Sci Med Sci 2003;58:82-91.
- Pollack LA, Greer GE, Rowland JH, Miller A, Doneski D, Coughlin SS, et al.
Cancer survivorship: a new challenge in comprehensive cancer control. Cancer Causes Control 2005;16 Suppl 1:51-9.
- A national action plan for cancer survivorship: advancing public health strategies. Atlanta (GA): US Department of Health and Human Services, Centers for Disease Control and Prevention; 2004.
- Hewitt M, Greenfield S, Stovall E, editors. From
cancer patient to cancer survivor: lost in transition. Committee on Cancer
Survivorship: Improving Care and Quality of Life. Washington (DC): National
Academies Press; 2006.
- National Comprehensive Cancer
Control Program. Centers for Disease Control and Prevention. http://www.cdc.gov/cancer/ncccp/about.htm. Accessed November 2, 2008.
- Measuring healthy days. Atlanta (GA): Centers for Disease Control and
Prevention; 2000.
- Guide to clinical preventive services. Agency for
Healthcare Research and Quality. http://www.ahrq.gov/clinic/cps3dix.htm#cancer. Accessed
September 30, 2009.
- Schag CA, Ganz PA, Wing DS, Sim MS, Lee JJ.
Quality
of life in adult survivors of lung, colon, and prostate cancer. Qual Life Res 1994;3(2):127-41.
- Richardson LC, Townsend JS, Fairley TL, Steele CB, Shah S, Woldman RL. Using the Behavioral Risk Factor Surveillance System (BRFSS) to
characterize cancer survivors. Poster presented at: Behavioral Risk Factor
Surveillance System Annual Conference; March 2008; Orlando, Florida.
- Richardson LC, Wingo PA, Zack MM, Zahran HS, King JB.
Health-related quality of life in cancer survivors between ages 20 and 64 years: population-based estimates from the Behavioral Risk Factor Surveillance System. Cancer 2008;112(6):1380-9.
- Sabatino SA, Coates RJ, Uhler RJ, Pollack LA, Alley LG, Zauderer LJ.
Provider counseling about health behaviors among cancer survivors in the United States. J
Clin Oncol 2007;25(15):2100-6.
- Sunga AY, Eberl MM, Oeffinger KC, Hudson MM, Mahoney MC.
Care of cancer survivors. Am Fam Physician 2005;71(4):699-706.
- Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al.
Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J
Clinical Oncol 2006;24(22):3535-41.
- Earle CC, Neville BA.
Under use of necessary care among cancer survivors. Cancer 2004;101(8):1712-9.
- Beuther DA, Sutherland ER.
Overweight,
obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med
2007;175(7):661-6.
- Alley DE, Chang VW.
The changing
relationship of obesity and disability, 1988-2004. JAMA 2007;298(17):2020-7.
- Bellizzi KM, Rowland JH, Jeffery DD, McNeel T.
Health
behaviors of cancer survivors: examining opportunities for cancer control
intervention. J Clin Oncol 2005;23(34):8884-93.
- Nathan PC, Greenberg ML, Ness KK, Hudson MM, Mertens AC, Mahoney MC, et al.
Medical care in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol 2008;26(27):4401-9.
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al.
Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm
Rep 2008;57(RR-7):1-60.
- Avritscher EBC, Cooksley CD, Geraci JM, Bekele BN, Cantor SB, Rolston
KV, et al.
Cost-effectiveness of influenza vaccination in working-age cancer patients. Cancer 2007;109(11):2357-64.
- McBean AM, Yu X, Virnig BA. Recommended healthcare among elderly cancer
survivors. J Clin Oncol (Meeting Abstracts) 2006;24(18 Suppl):6045.
- Blumberg SJ, Luke JV, Cynamon ML.
Telephone coverage and health survey estimates: evaluating the need for concern about wireless substitution. Am
J Public Health 2006;96(5):926-31.
- Desai MM, Bruce ML, Desai RA, Druss BG.
Validity of self-reported cancer history: a comparison of health interview data and cancer registry records. Am
J Epidemiol 2001;153(3):299-306.
- Nelson DE, Powell-Griner E, Town M, Kovar MG.
A comparison of national estimates from the National Health Interview Survey and the Behavioral Risk Factor Surveillance System. Am
J Public Health 2003;93(8):1335-41.
- Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, et al.
Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA 2003;290(12):1583-92.
- Massachusetts Health Care Reform 2007/2008 Progress Report. Commonwealth Health Insurance Connector Authority.
http://archives.lib.state.ma.us/bitstream/handle/ 2452/40954/ocn425961768.pdf?sequence=1. Accessed June 18, 2009.
Back to top
|
|
