Resources for Health Professionals
Diagnosis
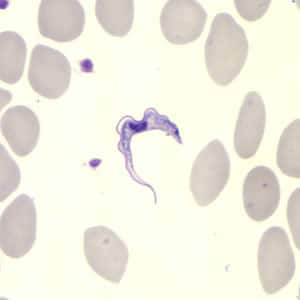
Definitive diagnosis rests on the observation of trypanosomes by microscopy.
In T. b. rhodesiense infection, the identification of suspected cases relies on the clinical presentation and a history of exposure. The level of parasitemia is relatively high, particularly in the first stage of disease, and trypanosomes can be found in blood. In centrifuged blood, the parasite sediments just above the white blood cells, and examination of buffy coat will increase sensitivity. Slides stained with Giemsa can be used, but it is easiest to find the parasite by microscopic examination of fresh wet preparations, because the trypanosomes are motile. Delay between sampling and microscopy should be minimized, because trypanosomes will lose motility within a few hours. Parasites can also be found in fluid expressed in trypanosomal chancres and in lymph node aspirates. Serologic testing is not used for the diagnosis of T. b. rhodesiense infection.
Detecting trypanosomes in T. b. gambiense infection is more difficult. The card agglutination test for trypanosomiasis/T. b. gambiense (CATT) is a serologic screening test used for population screening in endemic areas of Africa. It is not available in the United States. The test is insufficiently specific for confirmation of infection but can be helpful in identifying suspect cases. Parasitologic confirmation rests upon microscopic examination of chancre fluid or lymph node aspirate. The yield in lymph node examination varies from about 40% to 80%. Trypanosomes can also be found in blood, however, the yield is low, and concentration techniques (e.g., centrifugation followed by buffy coat examination, mini-anion exchange centrifugation technique, or microhematocrit centrifugation technique) are helpful. Serial examinations on consecutive days may be needed.
Staging for both T. b. gambiense and T. b. rhodesiense (i.e., assessment of neurological infection) is performed by microscopic examination of CSF collected by lumbar puncture on a wet preparation looking for motile trypomastigotes and WBCs. Patients with five or fewer WBCs per microliter and no trypomastigotes are considered to be in the first stage, and those with more than five WBCs per microliter or trypomastigotes are in the second stage. Other indications of second stage disease include elevated protein and an increase in nonspecific IgM in CSF.
Diagnostic assistance for African trypanosomiasis is available through DPDx.
Antitrypanosomal treatment is indicated for all persons diagnosed with African trypanosomiasis. Choice of therapy depends on the infecting subspecies of the parasite and on the disease stage. The first line drugs for both first and second stage disease are highly effective. Pentamidine is used to treat first stage T. b. gambiense infection in children < 6 years and < 20 kg without evidence of CNS disease. It is generally well-tolerated but may cause adverse reactions such as hypotension, hypoglycemia, injection site pain, diarrhea, nausea and vomiting. Fexinidazole is now available for treatment of first stage T. b. gambiense infection as an oral alternative in patients > 6 years weighing > 20kg. Suramin is also effective in treating the first stages of both T. b. gambiense and T. b. rhodesiense but is recommended to be only used to treat the first stage of T. b. rhodesiense because of the risk of severe adverse reactions in patients co-infected with onchocerciasis, which can occur in T. b. gambiense-endemic areas. Adverse reactions to suramin treatment in patients with T. b. rhodesiense trypanosomiasis are frequent, but usually mild and reversible. These include drug rash, nephrotoxicity, and peripheral neuropathy. In rare instances, suramin administration results in a hypersensitivity reaction, and, for this reason, a small test dose is usually given prior to the full first dose.
Second stage T. b. gambiense is treated with nifurtimox-eflornithine combination therapy (NECT) or fexinidazole (in patients > 6 years old weighing > 20 kg) depending on severity as measured by WBC in CSF and access to available regimens. There is limited data comparing fexinidazole to NECT but fexinidazole is preferred in low resource settings given the ease of administration as an oral drug. The NECT combination regimen appears to be more effective and less toxic than eflornithine monotherapy. Adverse events with eflornithine include fever, pruritus, hypertension, nausea, vomiting, diarrhea, abdominal pain, headaches, myelosuppression and more rarely, seizures. Eflornithine is not effective against T. b. rhodesiense and it is not recommended for treating the East African form of the disease.
Suramin is used to treat first stage T. b. rhodesiense infection. Melarsoprol, an organoarsenic compound, is the only drug available for treating second stage T. b. rhodesiense. Adverse reactions to melarsoprol can be severe and life-threatening. An encephalopathic reaction occurs in 5–10% of patients with a case-fatality rate of approximately 50% when it occurs. Prednisone or prednisolone is often given to patients who are being treated with melarsoprol to reduce the risk of encephalopathy. Other adverse reactions observed with melarsoprol include gastrointestinal and skin reactions, pyrexia, and peripheral neuropathy. Intravenous injections of melarsoprol are painful and can cause phlebitis.
Consultation with a subject matter expert is advised to discuss treatment options for patients with T. b. rhodesiense and T. b. gambiense infections. There is no test of cure for African trypanosomiasis. After treatment, patients should be closely followed for 24 months and monitored for relapse. Recurrence of symptoms will require examination of body fluids, including CSF, to detect the presence of trypanosomes.
| Species | Drug | Age Group | CSF Findings | Dosage and Duration | Comments |
| T. brucei gambiense, first stage |
Pentamidine* | < 6 years old or weight < 20 kg |
<5 WBC/uL, no trypanosomes | 4 mg/kg/day, IM or IV, (diluted in saline in 2-hour infusions) for 7 days. | |
| Fexinidazole♯ | > 6 years old and weight > 20 kg | Weight > 20 kg to < 35 kg: Loading dose 1,200 mg on days 1-4 Maintenance dose 600 mg on days 5-10–Weight > 35 kg: Loading dose 1,800 mg on days 1-4 Maintenance dose 1,200 mg on days 5-10 |
|||
| T. brucei gambiense, second stage | Nifurtimox^ and Eflornithine† combination (NECT) | < 6 years old or weight < 20 kg | >5 WBC/uL, or trypanosomes | Nifurtimox 15 mg/kg/day orally in three doses x 10 days and Eflornithine 400 mg/kg/day, IV, in two 2-hour infusions (each dose diluted in 250mL of water for injection) x 7 days. |
Eflornithine in children weighing <10 kg: dilute in 50 mL of water for injection. Children weighing 10–20 kg: dilute in 100 mL of water for injection. If water for injection is unavailable, eflornithine can be diluted in 5% dextrose or saline. Eflornithine might not be effective in immunosuppressed patients because it is trypanostatic and not trypanocidal. |
| Fexinidazole♯ | >6 years old and weight > 20 kg | < 100 WBC/uL | Weight > 20 kg < 35 kg: Loading dose 1,200 mg on days 1-4 Maintenance dose 600 mg on days 5-10–Weight > 35 kg Loading dose 1,800 mg on days 1-4 Maintenance dose 1,200 mg on days 5-10 |
||
| Nifurtimox^ and Eflornithine† combination (NECT) | >6 years old and weight > 20 kg | > 100 WBC/uL | Nifurtimox 15 mg/kg/day orally in three doses x 10 days and Eflornithine 400 mg/kg/day, IV, in two 2-hour infusions (each dose diluted in 250mL of water for injection) x 7 days. |
Eflornithine in children weighing 20–25 kg: dilute in 100 mL of water for injection. If water for injection is unavailable, eflornithine can be diluted in 5% dextrose or saline. Eflornithine might not be effective in immunosuppressed patients because it is trypanostatic and not trypanocidal. | |
| Trypanosoma brucei rhodesiense, first stage | Suramin | Test dose of 4-5 mg/kg (day 0) slowly IV, then 20 mg/kg IV (max 1 g/injection) over several hours, on days 1, 3, 7, 14, and 21. See comments for dosage in children |
Suramin in children (test dose): 2 mg/kg; maximum, 100 mg. Suramin in children (treatment dose): 10–20 mg/kg, maximum 1 g. In case of renal toxicity, consider alternative treatment schedules adjusting the daily dose and the interval between doses. | ||
| T. brucei rhodesiense, CNS involvement | Melarsoprol | 2.2 mg/kg/day (max 180-200mg/day), IV x 10 days | Corticosteroid pretreatment should be considered as it can reduce the risk of encephalopathic reaction to melarsoprol. |