Glycol Ethers 2-Methoxyethanol and 2-Ethoxyethanol
May 1983
DHHS (NIOSH) Publication Number 83-112
Current Intelligence Bulletin 39
The Glycol Ethers, with Particular Reference to 2-Methoxyethanol and 2-Ethoxyethanol: Evidence of Adverse Reproductive Effects
The National Institute for Occupational Safety and Health (NIOSH) recommends that 2-methoxyethanol (2ME) and 2-ethoxyethanol (2EE) be regarded in the workplace as having the potential to cause adverse reproductive effects in male and female workers. These recommendations are based on the results of several recent studies that have demonstrated dose-related embryotoxicity and other reproductive effects in several species of animals exposed by different routes of administration. Of particular concern are those studies in which exposure of pregnant animals to concentrations of 2ME of 2EE at or below their respective Occupational Safety and Health Administration (OSHA) permissible exposure limits (PEL’s) led to increased incidences of embryonic death, teratogenesis, or growth retardation. exposure of male animals resulted in testicular atrophy and sterility. In each case the animals had been exposed to 2ME or 2EE at concentrations at or below their respective Occupational Safety and Health Administration (OSHA) Permissible exposure limits (PEL’s). Therefore, appropriate controls should be instituted to minimize worker exposure to both compounds. NIOSH suggests that producers, distributors and users of 2ME and 2EE and of substances and materials containing 2ME and 2EE give this information to their workers and customers and that professional and trade associations and unions inform their members.
Background
Physical and Chemical Properties
The glycol ethers 2-methoxyethanol (2ME) and 2-ethoxyethanol (2EE) are part of a family of ethylene glycol ethers. At room temperature and atmospheric pressure, 2ME and 2EE are colorless liquids. Both compounds are completely miscible with water and with many organic solvents. Both are highly reactive in the presence of strong oxidizers; 2ME is also highly reactive in the presence of strong bases.1,2 Identifiers and synonyms for 2ME and 2EE are listed in Appendix II.
Production, Use, and Exposure
The Toxic Substances Control Act (TSCA) Inventory for 1977 reports a wide range of production volumes for 2ME (as many as 161 million pounds) and 2EE (as many as 171 million pounds).3
Both 2ME and 2EE are used as solvents in the manufacture of protective coatings such as lacquers, metal coatings, baking enamels, phenolic varnishes, epoxy resin coatings, and alkyd resins.1 They are also used as solvents for nitrocellulose, printing inks, textile dyes and pigments, and leather finishes. Both are used as anti-icing additives in brake fluids, in aviation fuels, and as antistall agents in gasoline. Both compounds are used in organic synthesis. In particular, a large amount of 2EE is used to manufacture 2-ethoxyethyl acetate (2EEA). 2EE is also used in the formulation of varnish removers, thinners, cleaning products, soaps, detergents, cosmetics, pesticides, pharmaceuticals, and adhesives. In addition to manufacturing operations, exposure to 2ME and 2EE may occur during the use of the many formulated products that contain them.
Based on the National Occupational Hazard Survey (NOHS) conducted by NIOSH between 1972 and 1974, it is estimated that as many as 100,000 workers are potentially exposed to 2ME and that 400,000 workers are potentially exposed to 2EE.4
Exposure Standards and Guides
OSHA’s Permissible Exposure Limit (PEL) for occupational exposure to 2ME is 25 ppm (80 mg/m3) and its PEL for 2EE is 200 ppm (740 mg/m3), both as a time-weighted average (TWA) for an 8-hour work shift (29 CFR 1910.1000).5 The OSHA standards bear a “Skin” notation, indicating the potential for skin absorption of toxic amounts of 2ME and 2EE. These standards are based primarily on reports of blood, kidney, liver, and central nervous system toxicity caused by 2ME and 2EE in animals and on case reports of human exposure to 2ME. No studies on reproductive effects of 2ME and 2EE were considered when these standards were adopted.
The American Conference of Governmental Industrial Hygienists (ACGIH) has recommended Threshold Limit Values (TLV’s) for 2ME and 2EE.6 The TLV for 2ME as a TWA for an 8-hour work shift is 25 ppm, and the Short Term Exposure Limit (STEL) for up to 15 minutes is 35 ppm. For 2EE, the ACGIH lowered its TLV in 1981 from 100 ppm to 50 ppm, and its STEL from 150 ppm to 100 ppm.7 The TLV for 2EE was lowered to prevent workers from being exposed to concentrations that had produced significant blood changes in laboratory animals.8 The ACGIH TLV’s also bear a “Skin” notation. In the Notice of Intended Changes (for 1982), TWA’s of 5 ppm are proposed for 2ME, 2EE, and their respective acetates; these intended changes are based primarily on testicular effects observed in recent animal studies.9
In 1982, most manufacturers of 2ME and 2EE adopted company industrial hygiene exposure guides below current OSHA PEL’s.10 For 2ME, the 8-hour TWA exposure limits range from 2-10 ppm. For 2EE, some manufacturers have adopted an 8-hour TWA of 5 ppm.
Effects of 2-Methoxyethanol (2ME)
Human Reproductive Effects
In one study of a small population involved in manufacturing and packaging of 2ME, no clinically significant differences were found between the exposed and comparison groups that could be attributed to the work environment for the fertility parameters studied. Exposures to 2ME were reported to be well below 25 ppm. 2EE and other glycol ethers were also manufactured in these facilities.11
Animal Studies
Exposure to 2ME caused dose-related adverse reproductive effects in female and male experimental animals. Pregnant mice exposed by gavage and pregnant rats and rabbits exposed by inhalation had increased incidences of embryonic deaths and abnormalities that were statistically significant (henceforth referred to as “significant”). Significantly increased incidences of testicular atrophy (decreased testicular weight) and microscopic testicular changes were observed among male mice given oral doses and male mice, rats, and rabbits exposed by inhalation. Infertility in male rats and abnormal sperm head morphology in mice have also been reported after inhalation of 2ME. Reproductive effects observed from exposure to 2ME are summarized in Table I. Most of the information contained in this Table as well as in Tables II and III is statistically significant. However, non statistically significant effects are also included in the tables when they were of the same or similar nature as those observed at higher doses in the same or a similar study. Tables I, II, and III also provide many of the details of the exposure conditions employed in the various studies; such detail is not provided in the text.
Female Animal Studies
Adverse effects in pregnant animals and their offspring after 2ME exposure that have been reported include: significant increases in embryonic deaths, major and minor fetal abnormalities, and maternal deaths and blood effects. Dose dependent embryo mortality and gross fetal defects were observed in fetuses of mice exposed by gavage to 2ME at 250 mg/kg on days 7-14 of gestation.12 Embryonic deaths were significantly increased among pregnant rabbits that inhaled 10 or 50 ppm of 2ME on days 6-18 of gestation. Similar results were reported after exposure at 3 ppm of 2ME, but they were not statistically significant.13 The authors of that study noted that the embryonic death rate in the rabbit control group was less than the rate observed in their historical rabbit control groups. The authors also noted that the rate of embryonic death among rabbits exposed at 10 ppm was comparable to the rate in historical controls. Nevertheless, embryonic mortality observed in this study did increase with the exposure concentration. No evidence of teratogenicity and only minimal fetotoxicity was observed in fetuses of rats exposed to 2ME at 50 ppm.13 However, another study did report fetal defects in rats after exposure at 50 ppm on days 7-15 of gestation.14 Fetal skeletal variations, which are among the most sensitive indicators of teratogenicity, were obtained at 2ME doses as low as 31 mg/kg/day given to pregnant mice.12 Male fetuses of pregnant mice exposed on days 6-15 of gestation at 50 ppm of 2ME had a significant increase in the incidence of unilateral testicular hypoplasia (underdevelopment of the testes).15
Unilateral testicular hypoplasia is considered a slight fetotoxic effect. Female rats and rabbits exposed to 2ME at 30, 100, or 300 ppm for 3 months had no evidence of gross reproductive or microscopic changes in the ovaries.16 No reduction of fertility was observed in the rats exposed at 300 ppm.17 In mice, embryonic deaths and fetal abnormalities occurred at lower 2ME doses than required to significantly lower maternal white blood cell (WBC) counts.12 A reduced WBC count was one basis for the current OSHA PEL for 2ME.
Male Animal Studies
Significant increases in testicular atrophy, microscopic testicular changes, and blood effects were reported in mature male animals exposed to 2ME. Mice given oral doses of 2ME at 250 mg/kg/day, 5 days/week for 5 weeks had severe testicular atrophy.18 Testicular atrophy and death occurred among rabbits after 13 weeks inhalation exposure to 2ME at 300 ppm; slight to severe microscopic testicular changes were observed at 30-100 ppm.16 In the same laboratory, no treatment related microscopic testicular changes were observed in rabbits exposed to 2ME at 3 ppm, 10 ppm or 30 ppm after a similar 13 week inhalation study.19 Testicular atrophy was observed in rats and mice exposed at 1,000 ppm of 2ME for 9 days.10. After 13 weeks of exposure at 300 ppm of 2ME, testicular atrophy16 and infertility were observed in rats.17 However, fertility was regained in 55% of the rats between weeks 26-32 of the study. Rats exposed for 5 days to 2ME at 500 ppm were temporarily infertile but fertility returned to control levels by week 10 after exposure.21 Mice similarly exposed to 2ME at 500 ppm developed abnormal sperm head morphology; the fertility of these mice was not tested.21 The testicular effects found in mice18 and rabbits16 occurred at lower doses of 2ME than those that caused significantly lower WBC counts.
Table I Reproductive Effects of 2-Methoxyethanol
| Sex | Species | Route of Administration & Dose | Effects | Reference |
|---|---|---|---|---|
| F, pregnant | Mouse | Gavage, 31-1,000 mg/kg, days 7-14 of gestation |
Embryonic death (100% at 1,000 mg/kg, 99.7% at 500 mg/kg, 53% at 250 mg/kg); fetal gross defects (250 mg/kg); skeletal malformations (62-250 mg/kg); lower fetal weight (125 & 250 mg/kg); fetal skeletal variations & delayed skeletal ossification (31-250 mg/kg) | 12 |
| F, pregnant | Rabit & Rat | Inhalation, 3-50 ppm, 6 hrs/day, gestation days 6-18 (rabbit) & days 6-15 (rat) | Embryonic death, rabbit (24% at 50 ppm & 11% at 10 ppm); major fetal external, skeletal & visceral abnormalities, lower fetal weight, rabbit (50 ppm); delayed skeletal ossification, rat and rabbit (50 ppm) | 13 |
| F, pregnant | Rat | Inhalation, 50-200 ppm, 7 hrs/day, days 7-15 of gestation | Embryonic death (200 ppm); fetal CV* & skeletal defects (50 & 100 ppm) | 14 |
| F, pregnant | Mouse | Inhalation, 3, 10 or 50 ppm, 6 hrs/day, days 6-15 of gestation |
Reduced litter size and fetal unilateral testicular hypoplasia (50 ppm) | 15 |
| F | Rabbit & Rat | Inhalation, 30-300 ppm, 6 hrs/day, 5 days/wk, 13 wks | No gross or microscopic changes in reproductive organs; death, rabbit (100 & 300 ppm) | 16 |
| F | Rat | Inhalation, 30-300 ppm, 6 hrs/day, 5 days/wk, 13 wks | No reduction of fertility | 17 |
| M | Mouse | Oral, 63-2,000 mg/kg, 5 days/wk, 5 wks | Testicular atrophy (250-2,000 mg/kg); death (2,000 mg/kg) | 18 |
| M | Rabbit & Rat | Inhalation, 30-300 ppm, 6 hrs/day, 5 days/wk, 13 wks | Testicular atrophy (300 ppm); microscopic testicular changes, rabbit (30-300 ppm), rat (300 ppm); death, rabbit (300 ppm) | 16 |
| M | Rabbit | Inhalation, 30-300 ppm, 6 hrs/day, 5 days/wk, 13 wks | No increase in gross or microscopic testicular changes | 19 |
| M | Rat | Inhalation, 30-300 ppm, 6 hrs/day, 5 days/wk, 13 wks | Infertility (300 ppm) | 17 |
| M | Rat & Mouse | Inhalation, 100-1,000 ppm, 6 hrs/day, 9 of 11 days | Testicular atrophy, rats and mice (1,000 ppm); microscopic testicular changes, rats (300 & 1,000 ppm) (No histopathology performed on mouse tissues.) | 20 |
| M | Rat | Inhalation, 25 or 500 ppm, 7 hrs/day, 5 days | Temporary infertility (500 ppm) | 21 |
| M | Mouse | Inhalation, 25 or 500 ppm, 7 hrs/day, 5 days | Abnormal spermhead morphology (500 ppm) | 21 |
*CV = Cardiovascular [return to table]
Mutagenicity Testing
The mutagenicity of 2ME was tested in Salmonella typhimurium strains TA 1535, TA 1537, TA 98, and TA 100 with and without Aroclor-induced rat liver S-9 supernatant.22 At the concentrations tested (up to 200 mg/plate), 2ME was not mutagenic.
Effects of 2-Ethoxyethanol (2EE)
Human Reproductive Effects
The only known published investigation of reproductive performance in a human population exposed to 2EE is difficult to interpret and is of questionable value because of mixed solvent exposures. Syrovadko and Malsheva evaluated the incidence of gynecological disorders and birth defects in female enameling workers.23 The two solvent mixtures used were chlorobenzene and 2EE (1:1), and tricresol and “solvent naptha” (1:4). The concentrations of 2EE were reported to have been “low.” There was no difference in the incidence of gynecological disorders between the enamelers and administrative workers (a comparison group including some former enamelers), but both groups were said to have 2.6-9.4 times more gynecological disorders than three other comparison groups. Among the disorders detected were inflammations, benign neoplasms, cervical erosions, and menstrual disorders. The rate of birth defects was significantly increased among the offspring of enamelers (10.0% vs 3.9% in plant controls), with heart and foot defects being predominant.
Animal Studies
Exposure of female and male animals to 2EE has caused significant dose-related adverse reproductive effects similar to the effects caused by 2ME. Oral, inhalation, subcutaneous, and dermal treatment of pregnant rats with 2EE caused increased incidences of embryonic death and abnormalities. 2EE inhalation exposure of pregnant rabbits and oral exposure of pregnant rats caused the same effects. The offspring of pregnant rats exposed by inhalation had altered behavior and neurochemical concentrations in the brain. In male mice, rats, and dogs treated orally and in rats treated subcutaneously, testicular atrophy and microscopic testicular changes have been reported. Table II summarizes the reproductive effects observed in animals exposed to 2EE.
Female Animal Studies
Significant increases in embryonic deaths, fetal abnormalities, altered behavioral test results, and changes in brain neurochemical concentrations have been reported after exposure of pregnant animals to 2EE. Embryonic deaths occurred in: rats after 2EE was given orally at 47 mg/kg/day;24 rabbits that inhaled 2EE at 160 ppm;25 rats that inhaled 2EE at 765 ppm;25 and rats that received 1.0 ml/day of 2EE dermally.26,27 The resorption rate was 100% in rats receiving 2.0 ml/day.26 Fetal cardiovascular and skeletal effects were found after inhalation exposure of pregnant rabbits at 160 ppm and pregnant rats at 200 ppm.25 Rabbit fetuses also had kidney and ventral body wall defects. Skeletal defects were detected in fetuses of rats that received 2EE at 93 mg/kg/day orally or subcutaneously,24 and 1.0 ml/day dermally.26 Fetal growth retardation indicated by lower weights and shorter lengths was observed in rats exposed by inhalation.25 Subtle teratogenic effects were reported in the offspring of pregnant rats exposed during gestation to 2EE at 100 ppm.28 The changes included altered behavioral test results at different stages of development after birth and differences in brain neurochemical concentrations in newborn and 21-day-old rats; these differences were more pronounced in the offspring exposed earlier in gestation. Maternal toxicity was greater in rabbits that inhaled 615 ppm of 2EE than in rats that inhaled 765 ppm.25 Rats receiving 2.0 ml/day dermally of 2EE exhibited a temporary lack of muscular coordination immediately after application.26 Exposure of female rats to 2EE at 650 ppm for three weeks prior to mating had no effect on fertility.25
Male Animal Studies
Significant increases in testicular atrophy, microscopic testicular changes, and deaths were reported in animals exposed to 2EE. Severe testicular atrophy occurred in mice given 1,000 mg/kg/day orally.18 Testicular changes were found in rats and dogs given 2EE at 186 mg/kg/day orally and in rats given 372 mg/kg/day subcutaneously.24 Testicular atrophy and blood effects in 2EE-treated mice were less severe than those in mice given 2ME at the same dosage levels; testicular effects in 2EE-treated mice occurred at a lower dose than the dose causing WBC effects.18
Mutagenicity Testing
The mutagenicity of 2EE was tested in Salmonella typhimurium strains TA 1535, TA 1537, TA 98, and TA 100 with and without Aroclor-induced rat liver S-9 supernatant.22 At the concentrations tested (up to 23 mg/plate), 2EE was not mutagenic. The National Toxicology Program reported that 2EE was not mutagenic in Salmonella typhimurium at 10 mg/plate.29 The same four Salmonella strains were used with and without microsomal fractions prepared from Aroclor-induced rat and hamster liver.
Carcinogenicity Testing
The Department of Health and Human Services’ National Toxicology Program is currently testing 2EE for carcinogenicity in male and female rats and mice at 0.5, 1.0, and 2.0 g/kg/day by gavage.29 Because mortality was high in the 2.0 g/kg groups, survivors were killed after 16 weeks; males had testicular lesions. The final report of this study should be available in 1983.
Table II Reproductive Effects of 2-Ethoxyethanol
| Sex | Species | Route of Administration & Dose | Effects | Reference |
|---|---|---|---|---|
| F, pregnant | Rat | Oral, 12-372 mg/kg/day, days 1-21 of gestation | Embryonic death increased (47-372 mg/kg); fetal skeletal defects & lower weight (93-186 mg/kg) | 24 |
| F, pregnant | Rat | sc*, 23-93 mg/kg/day, days 1-21 of gestation | Fetal skeletal defects & lower weight (93 mg/kg) | 24 |
| F. pregnant | Mouse | sc*, 47 or 93 mg/kg/day, days 1-18 of gestation | No embryotoxic or teratogenic effects | 24 |
| F, pregnant | Rabbit | sc*, 23 mg/kg/day, days 7-16 of gestation | No embryotoxic or teratogenic effects | 24 |
| F, pregnant | Rabbit | Inhalation, 160 or 615 ppm, 7 hrs/day, days 1-18 of gestation | Embryonic death (100% at 615 ppm & 22% at 160 ppm); fetal CV*, renal, and ventral body wall defects and skeletal variations (160 ppm); reduced maternal food consumption (160 & 615 ppm); maternal death (615 ppm) | 25 |
| F | Rat | Inhalation before pregnancy, 150 or 650 ppm, 7 hrs/day, 5 days/wk, 3 wks; then inhalation during gestation, 200 or 765 ppm, 7 hrs/day, days 1-19 of gestation | No effect on fertility
Embryonic death (100% at 765 ppm); fetal CV* & skeletal defects & growth retardation (200 ppm); mild maternal toxicity (765 ppm) |
25 |
| F, pregnant | Rat | Inhalation, 100 ppm, 7 hrs/day, days 7-13 or 14-20 of gestation | Altered behavioral test results; altered brain neurochemical concentrations | 28 |
| F, pregnant | Rat | Dermal, 1.0 or 2.0 ml/day, days 7-16 of gestation | Embryonic death (100% at 2.0 ml/day & 76% at 1.0 ml/day); fetal CV* defects & skeletal variations (1.0 ml/day) | 26 |
| M | Rat | Oral, 46-744 mg/kg, daily, 13 wks | Microscopic testicular changes (186 & 744 mg/kg) | 24 |
| M | Rat | sc*, 93-744 mg/kg, daily, 4 wks | Microscopic testicular changes (372 & 744 mg/kg) | 24 |
| M | Dog | Oral, 46-186 mg/kg, daily 13 wks | Microscopic testicular changes (186 mg/kg) | 24 |
| M | Mouse | Oral, 500-4,000 mg/kg, 5 days/wk, 5 wks | Testicular atrophy (1,000 & 2,000 mg/kg); death (4,000 mg/kg) | 18 |
CV = cardiovascular
sc = subcutaneous
Other Acute and Chronic Toxic Effects of 2ME and 2EE
The acute toxic effects of 2ME in humans are irritation of the eyes, nose, and throat; drowsiness; weakness; and shaking.1 Swallowing of 2ME may be fatal. Prolonged or repeated exposure may cause headache, drowsiness, weakness, fatigue, staggering, personality change, and decreased mental ability. In a 1978 case study report of two workers exposed to 2ME, Ohi and Wegman described clinical evidence of encephalopathy in both, bone marrow depression in one and pancytopenia in the other. Although airborne concentrations (8 ppm) were well below the PEL, both workers had significant skin contact with 2ME. The health status of both workers returned to normal after removal from exposure and treatment.30
In animals, 2EE has caused liver, kidney and lung damage, and anemia as well as eye irritation.
Effects of Other Structurally Related Glycol Ethers
Although there is limited experimental information on the reproductive effects of individual compounds structurally related to 2ME and 2EE, much of the information that is available is consistent with the reproductive effects caused by 2ME and 2EE. Table III summarizes the information on the eight structurally related glycol ethers discussed here: 2-methoxyethyl acetate (2MEA), 2-ethoxyethyl acetate (2EEA), 2-butoxyethanol(2BE), 2-phenoxyethanol (2PE), ethylene glycol dimethyl ether (EGdiME), bis(2-methoxyethyl) ether or diethylene glycol dimethyl ether (bis2ME), 2-(2-ethoxyethoxy)ethanol (2EEE), and 1-methoxy-2-propanol or propylene glycol monomethyl ether (IMP). The chemical structure of each compound is given in Appendix III. Seven of these compounds are ethylene glycol ethers, and one is a propylene glycol ether. These compounds share a similar stereochemical configuration. Some were tested only in male animals or only in female animals.
The acetate esters of 2ME and 2EE (2MEA and 2EEA, respectively) have caused male reproductive toxicity made equivalent to that of 2ME and 2EE in male mice. 2EEA appears to have fetoxicity and teratogenicity equivalent to that of 2EE in rats.14,27 Although additional studies are being conducted by NIOSH and others, the present information is insufficient to fully assess the potential for adverse reproductive effects on humans due to exposure to 2BE, 2PE, EGdiME, bis2ME, 2EEE, or 1MP. Nevertheless, some of this information is provided here so that the reader is alerted to their potential for causing adverse effects.
Table III Reproductive Effects of Glycol Ethers Structurally Related to 2-Methoxyethanol and 2-Ethoxyethanol
| Compound | Sex | Species | Route and Dosage | Effects | Reference |
|---|---|---|---|---|---|
| 2MEA | M | Mouse | Oral, 63-2,000 mg/kg, 5 days/wk, 5 wks | Testicular atrophy (500 – 2,000 mg/kg); death (2,000 mg/kg) | 18 |
| 2EEA | F, pregnant | Rat | Inhalation, 129-600 ppm, days 7-14 of gestation | Embryonic death (100% at 600 ppm); embryonic death (17% vs 4% controls) fetal CV & skeletal defects (387 ppm) | 14 * |
| F, pregnant | Rat | Dermal, 1.4 ml/day, days 7-16 of gestation | Embryonic death | 27 * | |
| M | Mouse | Oral, 500-4,000 mg/kg, 5 days/wk, 5 wks | Testicular atrophy (1,000-4,000 mg/kg); death (4,000 mg/kg) | 18 | |
| 2BE | F, pregnant | Rat | Inhalation, 200 ppm, days 7-14 of gestation | Slight maternal toxicity; no embryonic or teratogenic effects | 14* |
| F, pregnant | Rat | Dermal, 0.48 or 1.4 ml/day, days 7-16 of gestation | Maternal death (1.4 ml/day); no apparent excess embryonic death (0.48 ml/day) | 27* | |
| M | Mouse | Oral, 500-2,000 mg/kg, 5 days/wk, 5 wks | Microscopic testicular changes in 1 of 5 mice (1,000 mg/kg); death (2,000 mg/kg) | 18 | |
| 2PE | M | Mouse | Oral, 500-2,000 mg/kg, 5 days/wk, 5 wks | Microscopic testicular changes in 1 of 5 mice (1,000 mg/kg); death (2,000 mg/kg) | 18 |
| EGdiME | F, pregnant | Mouse | Gavage, 250-490 mg/kg, days 7-10 of gestation | Embryonic death (480 mg/kg); major fetal external and skeletal effects (350 & 490 mg/kg); fetal skeletal variations and growth retardation. | 32 |
| bis2ME | M | Rat | Inhalation, 250 or 1,000 ppm, 7 hrs/day, 5 days | Temporary infertility (1,000 ppm) | 32 |
| M | Mouse | Inhalation, 250 or 1,000 ppm, 7 hrs/day, 5 days | Abnormal spermhead morphology (1,000 ppm) | 32 | |
| 2EEE | F, pregnant | Rat | Inhalation, 100 ppm, days 7-15 of gestation | No embryotoxic or teratogenic effects | 14* |
| F, pregnant | Rat | Dermal, 1.4 ml/day, days 7-16 of gestation | No excess embryonic death | 27* | |
| 1MP | M | Rat & Mouse | Inhalation, 300-3,000 ppm, 6 hrs/day, 9 of 11 days | No microscopic testicular changes | 20 |
CV = Cardiovascular [return to table]
*Interim results; final results await skeletal and visceral evaluation [return to table]
EGdiME orally administered on days 7-10 gestation caused embryonic death and was teratogenic in mice.31 Preliminary data showed maternal death upon dermal application of 1.4 ml per day of 2BE to rats during gestation.27 The same preliminary investigation found no significant excess embryonic death upon dermal application of 0.48 ml per day of 2BE or 1.4 ml per day of 2EEE to rats during gestation; final results await skeletal and visceral evaluation.27 Microscopic evaluation revealed atrophy of the seminiferous tubules in one of five mice orally dosed with 2BE or 2PE.18 Inhalation exposure to bis2ME caused temporary infertility in rats and abnormal sperm head morphology in mice.32 No testicular effects were observed following, short-term IMP exposure as were observed from 2ME exposure in the same investigation.20
Conclusions and Recommendations
2ME and 2EE have caused significant increases of adverse reproductive effects in experimental animals of both sexes. 2ME was teratogenic and embryotoxic when administered to pregnant mice, rats, and rabbits. In non-pregnant female animals, 2ME caused no changes in reproductive organs that were discernible either grossly or microscopically. A single study indicated that the fertility of female rats was not affected by 2ME exposure. In male animals, exposure to 2ME resulted in testicular atrophy, histopathological testicular changes, infertility, and abnormal spermhead morphology. 2EE caused similar reproductive effects in animals. 2EE was teratogenic and embryotoxic when administered to pregnant rats and rabbits. In non-pregnant female rats, exposure to 2EE did not affect fertility, but it did produce varying degrees of toxicity in pregnant rabbits and rats. In males, 2EE produced testicular atrophy in mice and microscopic testicular changes in mice, rats, and dogs.
Adverse reproductive effects in both male and female animals have been reported at concentrations that ranged from less than to four times the OSHA PEL for 2ME or 2EE. Of particular concern are the changes observed below the OSHA PEL’S. Reproductive effects have been observed in both male and female animals exposed to 2ME at concentrations lower than those causing abnormal blood effects (low WBC counts). Although humans and animals may differ in their susceptibility to specific chemical compounds, any substance that produces adverse reproductive effects in animals should be considered to have the potential to cause similar reproductive effects in humans. This concern is highlighted by the fact that these effects have been observed in several species of animals exposed by various routes. As described above, exposure to 2ME has been associated with encephalopathy and pancytopenia in humans who had significant skin contact with this solvent. A desire to protect workers from such blood disturbances formed the basis, in part, for the current OSHA PEL’S.
Based on these recent findings, as well as continued concern for adverse effects after percutaneous absorption, NIOSH recommends that 2ME and 2EE be regarded in the workplace as having the potential to cause adverse reproductive effects in male and female workers and embryotoxic effects, including teratogenesis, in the offspring of the exposed, pregnant female.
Although there have been animal studies conducted at concentrations at which these effects did not occur, we cannot assume that these are safe concentrations for humans. By decreasing exposure the potential for adverse reproductive and embryotoxic effects will decrease.
Even though reproductive and embryotoxic risks have not been determined for workers exposed to 2ME and 2EE at their respective OSHA PEL’S, NIOSH believes that these standards should be reexamined. The adverse reproductive and embryotoxic potentials of 2ME and 2EE were not known when OSHA adopted these standards to protect workers against other acute and chronic effects.
NIOSH urges employers to voluntarily assess how their workers may be exposed to 2ME and 2EE and to reduce exposure to the lowest extent possible. The voluntary lowering of industrial exposure guides for 2ME and 2EE by some chemical manufacturers is commendable. The “Guidelines for Minimizing Worker Exposure to 2-Methoxyethanol and 2-Ethoxyethanol,” Appendix I, should be adapted to specific work situations.
As previously discussed, concern also extends to structurally related glycol ethers that have not been tested adequately to assess fully their potential for causing reproductive effects. Preliminary test results of some structurally related glycol ethers indicate that they also have the potential for causing adverse reproductive effects similar to 2ME and 2EE. In light of these findings, NIOSH recommends similar cautions be exercised to reduce worker exposure to these structurally related glycol ethers until adequate testing demonstrates their safety.
[signature]
J. Donald Millar, M.D.
Assistant Surgeon General
Director, National Institute for
Occupational Safety and Health
References
- National Institute for Occupational Safety and Health/Occupational, Safety and Health Administration: NIOSH/OSHA Occupational Health Guidelines for Chemical Hazards, DHHS (NIOSH) Publication No. 81-123 (1981).
- National Institute for Occupational Safety and Health/Occupational Safety and Health Administration: NIOSH/OSHA Pocket Guide to Chemical Hazards, DHEW (NIOSH) Publication No. 78-210, pp 94-95,128-129 (third printing, August 1980).
- Environmental Protection Agency, Office of Toxic Substances: Toxic Substances Control Act, Chemical Substances Initial Inventory — 1977. 2-Ethoxyethanol and 2-Methoxyethanol, by Manufacturer, January 4, 1982 (printout).
- National Institute for Occupational Safety and Health: National Occupational Hazard Survey. 2-Ethoxyethanol, 2-Methoxyethanol, Projected Numbers by Industry, October 9, 1982 (printout).
- U.S. Department of Labor, Occupational Safety and Health Administration: General Industry Standards, Publication 2206, 29 CFR 1910.1000, Washington, DC, 1981.
- TLV Airborne Contaminants Committee: Threshold Limit Values for Chemical Substances and Physical Agents in the Workroom Environment with Intended Changes for 1982. American Conference of Governmental Industrial Hygienists, Cincinnati, OH (1982).
- CTLV Airborne Contaminants Committee: Threshold Limit Values for Chemical Substances and Physical Agents in the Workroom Environment with Intended Changes for 1981. American Conference of Governmental Industrial Hygienists, Cincinnati, OH (1981).
- American Conference of Governmental Industrial Hygienists: Documentation of the Threshold Limit Values, 4th Ed., pp. 171-172, 259, Cincinnati, OH (1980).
- 9. Supplemental Documentation for 1982. American Conference of Governmental Industrial Hygienists, Cincinnati, OH (1982).
- Written communication. Submitted to NIOSH by G.V. Cox of the Chemical Manufacturers Association, (October 29, 1982).
- Cook, R.R., K.M. Bodner, R.C. Kolesar, P.F.D. Van Peenen, G.S. Dickson, K. Flanagan: A Cross-Sectional Study of Ethylene Glycol Monomethyl Ether Process Employees. Arch. Environ. Health 37(6):346-351 (1982).
- Nagano, K., E. Nakayama, H. Oobayashi, T. Yamada, H. Adachi, T. Nishizawa, H. Ozawa, M. Nakaichi, H. Okuda, K. Minami and K. Yamazaki: Embryotoxic Effects of Ethylene Glycol Monomethyl Ether in Mice. Toxicology 20:335-343 (1981).
- Hanley, T.R. Jr., B.L. Yano, K.D. Nitschke, and J.A. John: Ethylene Glycol Monomethyl Ether: Inhalation Teratology Study in Rats and Rabbits. Toxicology Research Laboratory, Dow Chemical U.S.A., Midland, MI (September 20, 1982).
- Nelson, B.K., J.V. Setzer, W.S. Brightwell, P.R. Mathinos, M.H. Kuczuk, and T.E. Weaver: Comparative Inhalation Teratogenicity of Four Industrial Glycol Ether Solvents in Rats. Abstract in Teratology 25:64A (1982). Corrected 2-ethoxyethylacetate concentrations and new data in teratology study. B.K. Nelson (March 25, 1982).
- Written communication. Interim report on ethylene glycol monomethyl ether submitted by E.H. Blair of the Dow Chemical Company to the Environmental Protection Agency under Section 8(e) of the Toxic Substances Control Act (January 29, 1982).
- Miller, R.R., J.A. Ayres, and J.T. Young: Ethylene Glycol Monomethyl Ether: 13-Week Vapor Inhalation Study with Rats and Rabbits. In press. Fundam. Appl. Toxicol.
- Rao, K.S., S.R. Cobel-Geard, J.T. Young, T.R. Hanley Jr., W.C. Hayes, J.A. John, and R.R. Miller: Ethylene Glycol Monomethyl Ether: Inhalation Reproduction and Dominant Lethal Study in Rats. Toxicology Research Laboratory, Dow Chemical U.S.A., Midland, MI, (February 10, 1982).
- Nagano, K., E. Nakayama, Me Koyano, H. Oobayaski, H. Adachi and T. Yamada: Mouse Testicular Atrophy Induced by Ethylene Glycol Monoalkyl Ethers. Jap. J. Ind. Health 21:29-35 (1979).
- Miller, R.R., L.L. Calhoun., and B.L. Yano: Ethylene Glycol Monomethyl Ether: 13-Week Vapor Inhalation Study with Male Rabbits, Toxicology Research Laboratory, Dow Chemical U.S.A., Midland, MI, (March 25, 1982).
- Miller, R.R., J.A. Ayres, L.L. Calhoun, J.T. Young and M.J. McKenna: Comparative Short-term Inhalation Toxicity of Ethylene Glycol Monomethyl Ether and Propylene Glycol Monomethyl Ether in Rats and Mice. Toxicol. Appl. Pharmacol. 61:368-377 (1981).
- McGregor, D. B.: Tier II Mutagenic Screening of 13 NIOSH Priority Compounds, Individual Compound Report on 2-Methoxyethanol. Report No. 22, submitted to NIOSH under Contract No. 210-78-0026. Inveresk Research International Limited, Musselburgh EH21 7UB, Scotland, 226 pp. (1980).
- Internal NIOSH communication. Ong, T.: Mutagenicity testing of 2-ethoxy/methoxy ethanol (September 29, 1980).
- Syrovadko, O.N. and Z.V. Malsheva: Work Conditions and Their Effect on Certain Specific Functions Among Women Who Are Engaged in the Production of Enamel-Insulated Wire. Gig . Tr. Prof. Zabol. (4):25-28 (1977).
- Stenger, E.G., L. Aeppli, D. Muller, E. Peheim, and P. Thomann: The Toxicology of Ethylene Glycol Monoethylether. Arzneim. Forsch. 21:880-885 (1971).
- Andrew, F.D., R.L. Buschbom, W.C. Cannon, R.A. Miller, L.F. Montgomery, D.W. Phelps, and M.R. Sikov: Teratologic Assessment of Ethylbenzene and 2-Ethoxyethanol. Submitted to NIOSH under Contract No. 210-79-0037. Battelle Pacific Northwest Laboratory, Richland, WA, 99 pp. (1981).
- Hardin, B.D., R.W. Niemeier, M.H. Kuczuk, P.R. Mathinos, and T.E. Weaver: Teratogenicity of 2-Ethoxyethanol by Dermal Application. Drug Chem. Toxicol. 5(3):277-294, (1982).
- Internal NIOSH communication. Hardin, B.D: Glycol Ether Comparative Skin Teratology (November 23, 1982).
- Nelson, B.K, W.S. Brightwell, J.V. Setzer, B.J. Taylor, R.W. Hornung and T.L. O’Donohue: Ethoxyethanol Behavioral Teratology in Rats. Neurotoxicology 2:231-249 (1981).
- Written communication. Submitted to NIOSH by R. Melnick of the National Toxicology Program (January 7, 1982).
- Ohi, G. and D.H. Wegman: Transcutaneous Ethylene Glycol Monomethyl Ether Poisoning in the Work Setting. J. Occup. Med. 20:675-76 (1978).
- Uemura, K.: The teratogenic effects of ethylene glycol dimethyl ether on the mouse. Acta Obstet. Gynaec. Jpn., 32(1):113-121, (1980).
- McGregor, D.B.: Tier II Mutagenic Screening of 13 NIOSH Priority Compounds, Individual Compound Report on Bis(2-methoxyethyl)ether. Report No. 34, submitted to NIOSH under Contract No. 210-78-0026. Inveresk Research International Limited, Musselburgh EH21 7UB, Scotland, 185 pp. (unpublished report, dated July 7, 1981).
- Leidel, N.A., K.A. Busch and J.R. Lynch: Occupational Exposure Sampling Strategy Manual. DHEW (NIOSH) Publication No. 77-173, 132 pp. (1977).
- National Institute for Occupational Safety and Health: NIOSH Manual of Analytical Methods, 2nd Ed., Vol. 2, P&CAM 79, pp. S79-1 to S79-8. DHEW (NIOSH) Publication No. 77-157-B (1977).
- National Institute for Occupational Safety and Health: NIOSH Manual of Analytical Methods, 2nd Ed., Vol. 5, P&CAM 361, pp. S361-1 to S361-10. DHEW (NIOSH) Publication No. 79-141 (1979).
- Hagopian, J.H. and E.K. Bastress: Recommended Industrial Ventilation Guidelines. DHEW (NIOSH) Publication No. 76-162, 330 pp. (1976).
- National Institute for Occupational Safety and Health: Registry for Toxic Effects of Chemical Substances, RTECS Master File Listing, Grids 0 07, C 09 (Microfiche) (January 1982).
APPENDIX I: Guidelines for Minimizing Worker Exposure to
2-Methoxyethanol and 2-Ethoxyethanol
NIOSH recommends that 2-methoxyethanol (2ME) and 2-ethoxyethanol (2EE) be regarded in the workplace as having the potential to cause adverse reproductive effects, including teratogenesis. Exposure should be limited to only those workers essential to the process or operation, and workplace exposure levels should be minimized. Less hazardous solvents should be substituted where practicable. Because there have been several studies that reported reproductive effects as a result of skin absorption, every effort should be made to eliminate skin exposure.
The guidelines listed below are general and have greatest application in industry; however, they should be given consideration for all settings and be adapted to specific situations.
Exposure Monitoring
Initial and routine worker exposure surveys should be made by competent industrial hygiene and engineering personnel. These surveys are necessary to determine the extent of worker exposure and to ensure that controls already in place are operational and effective. NIOSH’s Occupational Exposure Sampling Strategy Manual may be helpful in developing efficient programs to monitor worker exposure to 2ME and 2EE.33 The manual discusses how to determine the need for exposure measurements and select sampling times.
Worker exposures should be estimated by 8-hour TWA and short-term (15-minute) exposures calculated from personal or breathing zone samples. Short-term samples should be taken during periods of maximum expected exposure by using all available knowledge of the work areas, procedures, and processes. Area and source measurements may be useful to identify problem areas, processes, and operations.
Detailed analytical methods for both 2ME and 2EE are in the NIOSH Manual of Analytical Methods, Second Edition. The method for 2ME, #579, is in Volume 2,34 and the method for 2EE, #S361, is in Volume 5.35
Controlling Worker Exposure
There are four basic methods of limiting worker exposure to 2ME and 2EE, none of which is a simple industrial hygiene or management decision. Careful planning and thought should be used prior to implementation.
Product Substitution
The substitution of an alternative material with a lower potential health risk is an important method for reducing exposure. Extreme care must be used when selecting substitutes. Although the test results for some structurally related glycol ethers reported in this bulletin seem to suggest less hazardous compounds, the testing is not yet sufficient to identify a substitute for 2ME and 2EE. Possible health effects and potential exposures of alternatives to 2ME and 2EE should be fully evaluated prior to selection.
Contaminant Controls
Airborne concentrations of 2ME and 2EE can be most effectively controlled at the source of contamination by enclosure of the operation and use of local exhaust ventilation. Guidelines for selected processes and operations can be found in NIOSH’s Recommended Industrial Ventilation Guidelines.36 When enclosing a process or operation, a slight vacuum should be used to create negative pressure so that leakage will cause external air to flow into the enclosure and minimize contamination of the workplace. This can be accomplished with a well-designed local exhaust ventilation system that physically encloses the process as much as possible, with sufficient capture velocity to keep the contaminant from entering the workplace atmosphere. The design of ventilation systems should take into account the reactive characteristics of 2ME and 2EE.
Ventilation equipment should be checked at least every three months to ensure adequate performance. System effectiveness should also be checked soon after any change in production, process, or control that might result in significant increases in airborne exposure to 2ME and 2EE.
Worker Isolation
If feasible, workers may be isolated from direct contact with the work environment by the use of automated equipment operated from a closed control booth or room. The control room should be maintained at a greater air pressure than that surrounding the process equipment so that air flows out of, rather than into, the room. This type of control will not protect workers who must perform process checks, adjustments, maintenance, and related operations. Therefore, special precautions are often necessary to prevent or limit worker exposure in these situations and frequently involve the use of personal protective equipment.
Personal Protective Equipment
Personal protective equipment, which may include goggles, gloves, coveralls, footwear, and respirators, should not be the only means of preventing or minimizing exposure during routine operations. Since 2ME and 2EE can penetrate the skin, personal protective clothing and equipment should be selected that is impermeable to 2ME and 2EE.
Appendix II Identifiers and Synonyms for 2-Methoxyethanol and 2-Ethoxyethanol
- 2-MethoxyethanolChemical Abstracts Service Registry Number: 109-86-4
NIOSH RTECS Number: KL57750
Structural Formula: CH3-0-CH2-CH2-OH
Empirical Formula: C3H802Identifiers and Synonyms for 2-Methoxyethanol and 2-Ethoxyethanol Dowanol EM Methyl Cellosolve Ethylene glycol methyl ether Methyl glycol Ethylene glycol monomethyl ether Methyl oxitol Glycolmethyl ether Monomethyl ether of ethylene glycol MECS Poly-solv EM Methoxyhydroxyethane - 2-EthoxyethanolChemical Abstracts Service Registry Number: 110-80-5
NIOSH RTECS Number: KK80500
Structural Formula: CH3-CH2-0-CH2-CH2-OH
Empirical Formula: C4H10O2
| Cellosolve | Glycol ethyl ether |
| Cellosolve solvent | Glycol monoethyl ether |
| Dowanol EE | Hydroxy ether |
| Ethyl cellosolve | NCI-C54853 |
| Ethylene glycol ethyl ether | Oxitol |
| Ethylene glycol monoethyl ether | Poly-solv EE |
This information was obtained from the NIOSH’s computerized Registery of Toxic Effects of Chemical Substances (RTECS).37 Registered trademark information is not included in this file. Therefore, some of the above synonyms and identifiers have trademarks but are not so indicated.
Appendix III Structurally Related Glycol Ethers
| 2MEA | 2-Methoxyethyl acetate | 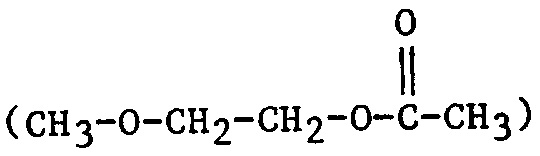 |
| 2EEA | 2-Ethoxyethyl acetate | 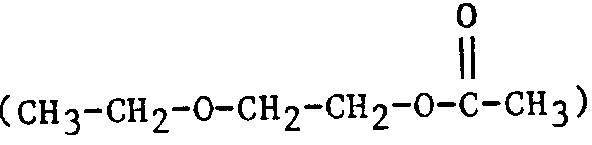 |
| 2BE | 2-Butoxyethanol | 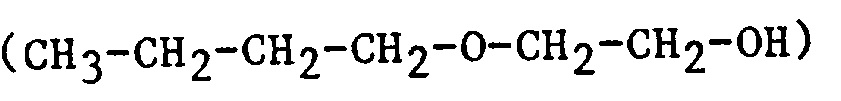 |
| 2PE | 2-Phenoxyethanol | 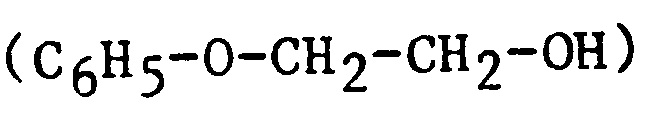 |
| EGdiME | Ethylene glycol dimethyl ether |
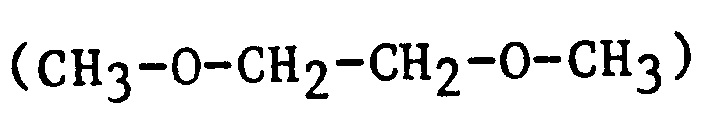 |
| bis2ME | bis(2-methoxyethyl)ether | 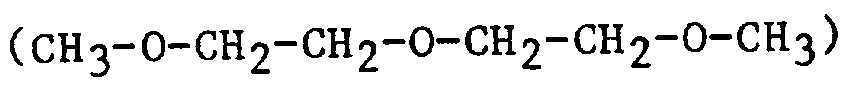 |
| 2EEE | 2-(2-Ethoxyethoxy)ethanol | 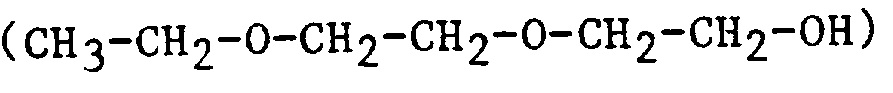 |
| 1MP | 1-Methoxy-2-propanol or propylene glycol monomethyl ether |
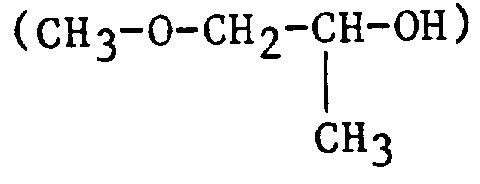 |
