Multistate Infestation with the Exotic Disease–Vector Tick Haemaphysalis longicornis — United States, August 2017–September 2018
Weekly / November 30, 2018 / 67(47);1310–1313
C. Ben Beard, PhD1; James Occi, MA, MS2; Denise L. Bonilla, MS3; Andrea M. Egizi, PhD4; Dina M. Fonseca, PhD2; James W. Mertins, PhD3; Bryon P. Backenson, MS5; Waheed I. Bajwa, PhD6; Alexis M. Barbarin, PhD7; Matthew A. Bertone, PhD8; Justin Brown, DVM, PhD9; Neeta P. Connally, PhD10; Nancy D. Connell, PhD11; Rebecca J. Eisen, PhD1; Richard C. Falco, PhD5; Angela M. James, PhD3; Rayda K. Krell, PhD10; Kevin Lahmers, DVM, PhD12; Nicole Lewis, DVM13; Susan E. Little, DVM, PhD14; Michael Neault, DVM15; Adalberto A. Pérez de León, DVM, PhD16; Adam R. Randall, PhD17; Mark G. Ruder, DVM, PhD18; Meriam N. Saleh, PhD14; Brittany L. Schappach10; Betsy A. Schroeder, DVM19; Leslie L. Seraphin, DVM3; Morgan Wehtje, PhD3; Gary P. Wormser, MD20; Michael J. Yabsley, PhD21; William Halperin, MD, DrPH22 (View author affiliations)
View suggested citationSummary
What is already known about this topic?
Haemaphysalis longicornis is a tick indigenous to Asia, where it is an important vector of human and animal disease agents, which can result in human hemorrhagic fever and substantive reduction in dairy production.
What is added by this report?
During 2017–2018, H. longicornis has been detected in Arkansas, Connecticut, Maryland, New Jersey, New York, North Carolina, Pennsylvania, Virginia, and West Virginia on various species of domestic animals and wildlife, and from two humans.
What are the implications for public health practice?
The presence of H. longicornis in the United States represents a new and emerging disease threat. Characterization of the tick’s biology and ecology are needed, and surveillance efforts should include testing for potential indigenous and exotic pathogens.
Haemaphysalis longicornis is a tick indigenous to eastern Asia and an important vector of human and animal disease agents, resulting in such outcomes as human hemorrhagic fever and reduction of production in dairy cattle by 25%. H. longicornis was discovered on a sheep in New Jersey in August 2017 (1). This was the first detection in the United States outside of quarantine. In the spring of 2018, the tick was again detected at the index site, and later, in other counties in New Jersey, in seven other states in the eastern United States, and in Arkansas. The hosts included six species of domestic animals, six species of wildlife, and humans. To forestall adverse consequences in humans, pets, livestock, and wildlife, several critical actions are indicated, including expanded surveillance to determine the evolving distribution of H. longicornis, detection of pathogens that H. longicornis currently harbors, determination of the capacity of H. longicornis to serve as a vector for a range of potential pathogens, and evaluation of effective agents and methods for the control of H. longicornis.
H. longicornis is native to eastern China, Japan, the Russian Far East, and Korea. It is an introduced, and now established, exotic species in Australia, New Zealand, and several island nations in the western Pacific Region. Where this tick exists, it is an important vector of human and animal disease agents. In China and Japan, it transmits the severe fever with thrombocytopenia syndrome virus (SFTSV), which causes a human hemorrhagic fever (2), and Rickettsia japonica, which causes Japanese spotted fever (3). Studies in Asia identified ticks infected with various species of Anaplasma, Babesia, Borrelia, Ehrlichia, and Rickettsia, and all of these pathogen groups circulate zoonotically in the United States (4,5). In addition, parthenogenetic reproduction, a biologic characteristic of this species, allows a single introduced female tick to generate progeny without mating, thus resulting in massive host infestations. In some regions of New Zealand and Australia, this tick can reduce production in dairy cattle by 25% (6). Before 2017, H. longicornis ticks were intercepted at U.S. ports of entry at least 15 times on imported animals and materials (James W. Mertins, U.S. Department of Agriculture [USDA], personal communication).
The USDA Animal and Plant Inspection Service coordinated cooperative efforts through telephone conference calls with various local, state, and federal agricultural and public health agencies. Through these efforts, enhanced vector and animal surveillance were implemented to detect additional tick infestations. Suspect archival specimens that were available among previously collected ticks were also examined. Ticks were identified definitively by morphology at the USDA National Veterinary Services Laboratories or by DNA sequence analysis (molecular barcoding) at Rutgers University Center for Vector Biology, Monmouth County (New Jersey) Mosquito Control Division; College of Veterinary Medicine, University of Georgia; and Center for Veterinary Health Sciences, Oklahoma State University. By definition, a “report” is any new morphologic or molecular identification of H. longicornis ticks with a new county or host species from that county, identified from August 2017 through September 2018. Subsequent repeat collections are not reported here.
From August 2017 through September 2018, vector and animal surveillance efforts resulted in 53 reports of H. longicornis in the United States, including 38 (72%) from animal species (23 [61%] from domestic animals, 13 [34%] from wildlife, and two [5%] from humans), and 15 (28%) from environmental sampling of grass or other vegetation using cloth drags or flags* or carbon dioxide–baited tick traps.† With the exception of one report from Arkansas, the remaining reports of positively identified ticks are from eight eastern states: New Jersey (16; 30%), Virginia (15; 28%), West Virginia (11; 21%), New York (three; 6%), North Carolina (three; 6%), Pennsylvania (two; 4%), Connecticut (one; 2%), and Maryland (one; 2%) (Figure). Among the 546 counties or county equivalents in the nine states, ticks were reported from 45 (8%) counties (1.4% of all 3,109 U.S. counties and county equivalents) (Table 1). Excluding 15 reports of positive environmental sampling using flagging, dragging, or carbon dioxide traps, the remaining 38 reports reflect collection of ticks from infested host species (Table 2). Surveillance efforts did not include testing the ticks or hosts for pathogens. No cases of illness in humans or other species were reported. Concurrent reexamination of archived historical samples showed that invasion occurred years earlier. Most importantly, ticks collected from a deer in West Virginia in 2010 and a dog in New Jersey in 2013 were retrospectively identified as H. longicornis.
Discussion
Cooperative efforts among federal, state, and local experts from agricultural, public health, and academic institutions during the last year have documented that a tick indigenous to Asia is currently resident in several U.S. states. The public health and agricultural impacts of the multistate introduction and subsequent domestic establishment of H. longicornis are not known. At present, there is no evidence that H. longicornis has transmitted pathogens to humans, domestic animals, or wildlife in the United States. This species, however, is a potential vector of a number of important agents of human and animal diseases in the United States, including Rickettsia, Borrelia, Ehrlichia, Anaplasma, Theileria, and several important viral agents such as Heartland and Powassan viruses. Consequently, increased tick surveillance is warranted, using standardized animal and environmental sampling methods.
The findings in this report are subject to at least two limitations. First, the findings are limited by the variable surveillance methods used to identify the geographic and host distribution of H. longicornis. These methods included both passive and active surveillance. Conclusions about the geographic and host distribution might reflect the biases in the collection and submission of samples to states and USDA and the paucity of available information. Second, the data in this report reflect the collection of specimens that were positively identified by morphology or molecular barcoding. These represent sentinels that H. longicornis is present in different U.S. states and regions, and not a comprehensive assessment of the distribution of H. longicornis in the United States. The absence of positive samples from many states and counties might reflect the absence of infestation, absence of sampling, or failure to recover the tick. Even in states where H. longicornis has been found, the available data do not describe the actual extent or intensity of infestation.
The biology and ecology of H. longicornis as an exotic species in the United States should be characterized in terms of its vector competence (ability to transmit a pathogen) and vectorial capacity (feeding habits, host preference, climatic sensitivity, population density, and other factors that can affect the risk for pathogen transmission to humans) for tickborne pathogens known to be present in the United States (5). Surveillance for H. longicornis should include adequate sampling of companion animals, commercial animals, wildlife, and the environment. Where H. longicornis is detected, there should be testing for a range of indigenous and exotic viral, bacterial, and protozoan tickborne pathogens potentially transmitted by H. longicornis. Given the similarity between SFTSV and Heartland virus, a tickborne phlebovirus (https://www.cdc.gov/heartland-virus/index.html), further evaluation of the potential role of H. longicornis in transmission of this disease agent among animal reservoirs and possibly to humans is warranted. A broad range of interventions should be evaluated, including insecticide and acaricide sensitivity testing. Many state and federal agencies are developing and disseminating information for stakeholders, including development of hotlines, and some states are identifying ticks submitted by the public. The recently documented occurrence of H. longicornis in the United States presents an opportunity for collaboration among governmental, agricultural, public health agencies and partners in academic public health, veterinary sciences, and agricultural sciences to prevent diseases of potential national importance before onset in humans and other animal species.
Acknowledgments
Wes Watson, Andrew D. Haddow, Naomi Drexler, Gleeson Murphy, Harry Savage, Howard Ginsberg, Kim Cervantes, field and laboratory personnel.
Corresponding author: C. Ben Beard, cbeard@cdc.gov, 970-221-6418.
1Division of Vector-Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases, CDC; 2Center for Vector Biology, School of Environmental and Biological Sciences, Rutgers, The State University of New Jersey, New Brunswick, New Jersey; 3Animal and Plant Health Inspection Service, Veterinary Services, U.S. Department of Agriculture, Riverdale, Maryland; 4Tick-borne Disease Laboratory, Monmouth County Mosquito Control Division, and Center for Vector Biology, Department of Entomology, Rutgers, The State University of New Jersey, New Brunswick, New Jersey; 5Bureau of Communicable Diseases Control, New York State Department of Health; 6New York City Department of Health and Mental Hygiene; 7Communicable Disease Branch, North Carolina Division of Public Health; 8Department of Entomology and Plant Pathology, North Carolina State University, Raleigh, North Carolina; 9Pennsylvania Game Commission, Animal Diagnostic Laboratory, Harrisburg, Pennsylvania; 10Department of Biological and Environmental Sciences, Western Connecticut State University, Danbury, Connecticut; 11Johns Hopkins Center for Health Security, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland; 12Virginia-Maryland College of Veterinary Medicine, Virginia Polytechnic Institute and State University, Blacksburg, Virginia; 13Division of Animal Health, New Jersey Department of Agriculture; 14Center for Veterinary Health Sciences, Oklahoma State University, Stillwater, Oklahoma; 15Veterinary Division, North Carolina Department of Agriculture and Consumer Services; 16Agricultural Research Service, Knipling-Bushland U.S. Livestock Insects Research Laboratory, U.S. Department of Agriculture, Kerrville, Texas; 17Animal and Plant Health Inspection Service, Wildlife Services, U.S. Department of Agriculture, Riverdale, Maryland; 18Southeastern Cooperative Wildlife Disease Study, Department of Population Health, University of Georgia, Athens, Georgia; 19Bureau of Epidemiology, Pennsylvania Department of Health, 20Department of Medicine, New York Medical College, Valhalla, New York; 21Department of Population Health, College of Veterinary Medicine, and the Warnell School of Forestry and Natural Resources, University of Georgia, Athens, Georgia; 22Department of Epidemiology, School of Public Health, Rutgers, The State University of New Jersey, New Brunswick, New Jersey.
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest. Susan E. Little reports grants, personal fees, and nonfinancial support from several veterinary pharmaceutical and diagnostic companies, outside the submitted work. Mark G. Ruder reports grants from U.S. Department of Agriculture during the conduct of the study and grants from U.S. Department of Agriculture, outside the submitted work. Gary P. Wormser reports unpaid board membership in the American Lyme Disease Foundation; fees for expert medical/legal testimony regarding Lyme disease and babesiosis; grants to New York Medical College from Immunetics, Inc., Quidel Corporation, and Rarecyte, Inc. for diagnostic tests for Lyme disease and babesiosis, Tufts University for xenodiagnoses to assess persistence of Borrelia, and Institute for Systems Biology for exploration of biomarkers for Lyme disease outcomes; U.S. Patent Application, “High Sensitivity Method for Early Lyme Disease Detection” (Application No. 15/046,204); and U.S. Provisional Patent Application, “Use of Metabolic Biosignatures for Differentiation of Early Lyme Disease from Southern Tick-Associated Rash Illness (STARI)” (Application No. 62/277,252); and stock/stock options in Abbott/AbbVie. No other potential conflicts of interest were disclosed.
* Drags consist of white cloth (usually 1 m2) that have a wooden leading frame and are dragged by a cord through grass or a leafy forest floor. Flags are similar but are used to brush uneven surfaces such as small bushes in wooded areas. Drags and flags are used to sample the environment for ticks trying to locate a host.
† Carbon dioxide traps consist of dry ice–filled small boxes with holes that allow the CO2 to escape which are placed on a white cloth or mat in a grassy area or forest floor. Ticks, attracted by the CO2, crawl on to the cloth or mat surface, which is inspected for ticks after a period of time.
References
- Rainey T, Occi JL, Robbins RG, Egizi A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J Med Entomol 2018;55:757–9. CrossRef PubMed
- Luo L-M, Zhao L, Wen H-L, et al. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg Infect Dis 2015;21:1770–6. CrossRef PubMed
- Mahara F. Japanese spotted fever: report of 31 cases and review of the literature. Emerg Infect Dis 1997;3:105–11. CrossRef PubMed
- Kang J-G, Ko S, Smith WB, Kim H-C, Lee I-Y, Chae J-S. Prevalence of Anaplasma, Bartonella and Borrelia species in Haemaphysalis longicornis collected from goats in North Korea. J Vet Sci 2016;17:207–16. CrossRef PubMed
- Rosenberg R, Lindsey NP, Fischer M, et al. Vital signs: trends in reported vectorborne disease cases—United States and territories, 2004–2016. MMWR Morb Mortal Wkly Rep 2018;67:496–501. CrossRef PubMed
- Heath A. Biology, ecology and distribution of the tick, Haemaphysalis longicornis Neumann (Acari: Ixodidae) in New Zealand. N Z Vet J 2016;64:10–20. CrossRef PubMed
 FIGURE. Counties and county equivalents* where Haemaphysalis longicornis has been reported (N = 45) — United States, August 2017–September 2018
FIGURE. Counties and county equivalents* where Haemaphysalis longicornis has been reported (N = 45) — United States, August 2017–September 2018
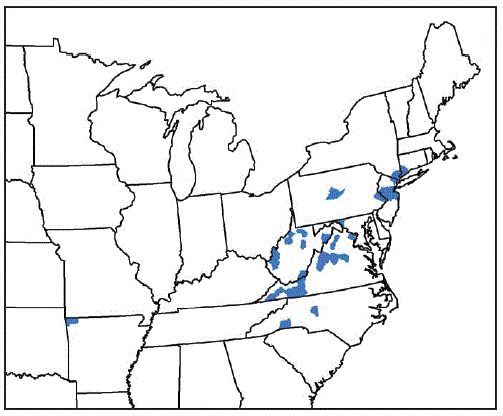
* Benton County, Arkansas; Fairfield County, Connecticut; Washington County, Maryland; Bergen, Hunterdon, Mercer, Middlesex, Monmouth, Somerset, and Union Counties, New Jersey; Davidson, Polk, and Rutherford Counties, North Carolina; Richmond, Rockland, and Westchester Counties, New York; Bucks and Centre Counties, Pennsylvania; Albemarle, Augusta, Carroll, Fairfax, Giles, Grayson, Louisa, Page, Pulaski, Rockbridge, Russell, Scott, Smyth, Staunton City, Warren, and Wythe Counties, Virginia; Cabell, Hardy, Lincoln, Mason, Marion, Monroe, Putnam, Ritchie, Taylor, Tyler, Upshur Counties, West Virginia.
* Counties or county equivalents
Suggested citation for this article: Beard CB, Occi J, Bonilla DL, et al. Multistate Infestation with the Exotic Disease–Vector Tick Haemaphysalis longicornis — United States, August 2017–September 2018. MMWR Morb Mortal Wkly Rep 2018;67:1310–1313. DOI: http://dx.doi.org/10.15585/mmwr.mm6747a3.
MMWR and Morbidity and Mortality Weekly Report are service marks of the U.S. Department of Health and Human Services.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services.
References to non-CDC sites on the Internet are
provided as a service to MMWR readers and do not constitute or imply
endorsement of these organizations or their programs by CDC or the U.S.
Department of Health and Human Services. CDC is not responsible for the content
of pages found at these sites. URL addresses listed in MMWR were current as of
the date of publication.
All HTML versions of MMWR articles are generated from final proofs through an automated process. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (https://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables.
Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.