 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Perspectives from the Dracunculiasis Eradication Programme*D.R. Hopkins** After a slow beginning in association with the International Drinking Water Supply and Sanitation Decade (1981-1990), the global Dracunculiasis Eradication Programme has reduced the incidence of dracunculiasis by nearly 97%, from an estimated 3.2 million cases in 1986 to less than 100,000 cases in 1997. Over half of the remaining cases are in Sudan. In addition, the programme has already produced many indirect benefits such as improved agricultural production and school attendance, extensive provision of clean drinking-water, mobilization of endemic communities, and improved care of infants. Most workers in the campaign have other responsibilities in their communities or ministries of health besides dracunculiasis eradication. Introduction Dracunculiasis (guinea-worm disease) is an infection in humans caused by the parasite Dracunculus medinensis, which is contracted by drinking contaminated water from ponds, step wells or other open stagnant sources. After about 1 year, the 0.6-0.9-m long adult female worm emerges slowly through the victim's skin in an attempt to deposit immature larvae in water. Some of the larvae are eaten by a tiny crustacean or copepod (Cyclops), in which the larvae undergo two moults within about 2-3 weeks. People are infected when they drink water containing the copepods with infective larvae. Each infection lasts 1 year, and there is no protective immunity. Humans are the only definitive hosts of D. medinensis, and they are infected only by drinking contaminated water. Once a person is infected, there is no treatment to kill the parasite before it emerges a year later. The disease can be prevented, however, by teaching people to filter their drinking-water through a finely woven cloth or to boil their water if they can afford it; by educating communities to keep people with emerging worms from entering sources of drinking-water; by applying the cyclopsicide temephos to contaminated sources every 4 weeks; or by providing safe sources of drinking-water from borehole wells (1). Dracunculiasis is rarely fatal, but the pain and secondary infections associated with the emerging worm incapacitate infected persons for periods averaging 8 weeks. The worms emerge on the lower leg and are the sole evidence of the infection; however, they may emerge from any part of the body, and a dozen or more may emerge simultaneously from some infected persons. Over half of a village's population may be infected at the same time, and the outbreaks usually coincide with the planting or harvest season and the school year. Thus the impact of this quintessentially rural disease manifests itself in mass temporary crippling, which in turn substantially reduces agricultural production and greatly increases school absenteeism (2). Other indirect adverse effects have been documented on infant nutrition, child care and childhood immunizations (3,4). The Eradication Campaign The global campaign to eradicate dracunculiasis began with an initiative at the Centers for Disease Control and Prevention (CDC) in 1980, which took advantage of the impending International Drinking Water Supply and Sanitation Decade (1981-1990) (5). It was not known how many people were infected by dracunculiasis at that time, but a WHO estimate put the number at about 10 million (6). In addition to India and Pakistan, 16 countries in sub-Saharan Africa were known to be infected (Benin, Burkina Faso, Cameroon, Chad, Cote d'Ivoire, Ethiopia, Ghana, Kenya, Mali, Mauritania, Niger, Nigeria, Senegal, Sudan, Togo, and Uganda). Yemen was discovered to be endemic in 1994. In 1986, Watts published a country-by-country estimate of the numbers of persons infected, which totalled 3.2 million (7). Over 120 million persons were judged to be at risk of the infection in Africa alone. Despite the adoption of dracunculiasis eradication in 1981 as a sub-goal of the Water and Sanitation Decade, one of the main goals of which was to provide safe drinking-water to all who did not yet have it, support for the eradication programme was exceedingly slow in coming. In 1982 the US National Research Council, CDC, and the US Agency for International Development convened an international Workshop on Opportunities for Control of Dracunculiasis in Washington in collaboration with WHO. In 1986, the World Health Assembly adopted its first resolution calling for the "elimination" of dracunculiasis; the first African Regional Conference on Dracunculiasis Eradication met in Niamey, Niger; and The Carter Center (Global 2000) and CDC began assisting the eradication programme in Pakistan. African ministers of health resolved at Brazzaville in 1988 to eradicate dracunculiasis by the end of 1995, a target date which was endorsed by the World Health Assembly in 1991. An international donors' conference co-sponsored by The Carter Center, UNDP and UNICEF at Lagos in 1989 mobilized US$ 10 million for the global programme. As illustrated elsewhere (8), however, by the end of the Water Decade, only four of the 18 endemic countries (India, Pakistan, Ghana, and Nigeria) had begun implementing national eradication programmes, and 10 of the countries only began implementing their programmes in 1993 or 1994. Much more was accomplished in the 1990s. As shown in Figure 1, the numbers of reported cases of dracunculiasis have been reduced by almost 97%, to less than 100,000 in 1997, as compared to the estimated 3.2 million cases in 1986, and the nearly one million cases which were actually reported in 1989. By the end of 1997, Pakistan had been certified by WHO as free of dracunculiasis, India had halted transmission of the disease, and Yemen, the only other known affected country in Asia, had found only seven cases in the entire year. In Africa, Kenya had reported no indigenous cases since May 1994, Cameroon had only one indigenous case since September 1996, and Senegal and Chad reported only 4 and 25 cases in 1997, respectively (Figure 2). Globally, the number of known endemic villages has been reduced from about 23,000 at the beginning of 1993, to less than 10,000 at the beginning of 1998, more than half of which are in Sudan. Remaining Challenges Over 90% of the remaining cases of dracunculiasis are restricted to parts of only five countries (Burkina Faso, Ghana, Niger, Nigeria and Sudan). Each of these five countries presents unique difficulties, but the most serious by far is the continuing civil war in southern Sudan, where access to some of the most highly endemic foci seen anywhere in the world is severely constrained, and where the national eradication programme has not yet had any access at all to several probably endemic areas. Surveillance and control measures were less complete in Sudan in 1997 than in 1996 because of increased strife in 1997. Although the target date for global eradication of dracunculiasis was not met, our goal now is to achieve eradication as soon as possible. Apart from the fighting in Sudan, the Dracunculiasis Eradication Programme (DEP) has suffered for many years, and continues to be plagued by opposing views held by some representatives of major partners in the campaign regarding the most appropriate strategy for implementing the programme. Some of these disagreements resulted from unrecognized differences in what was meant by "integration". When integration means that dracunculiasis eradication activities should be among the responsibilities of all health workers in a country's established public health network, wherever possible, that is entirely appropriate. That is also exactly the approach which has been used in the DEP from the beginning -- to mobilize and support otherwise underutilized members of existing health services at national, regional, and subregional levels. Those pre-existing health workers in turn supervise and support part-time village volunteers, most of whom were recruited by the DEP, because primary health care services had not reached these remote villages. Few of those health workers, and almost none of the village volunteers, are exclusively devoted to work on dracunculiasis. In Africa, 9 of the 15 national programme coordinators of Dracunculiasis Eradication Programmes have other responsibilities in their ministries of health besides dracunculiasis eradication. In south-east Nigeria, 8 of the 10 chairmen of the state task forces for guinea-worm eradication are the state directors of public health services, in charge of all primary health care services; at the local government area (LGA) level, all of the 25 LGA coordinators for the dracunculiasis programme are local government health officials who are responsible for other health programmes. In Izzi and Ebonyi LGAs of Ebonyi State, the most endemic state in Nigeria, 100% of the 348 village level workers in the programme are unpaid volunteers, mostly farmers, not full-time "vertical guinea-worm staff", including the 4% who are community health workers with other medical responsibilities. When the DEP began in south-east Nigeria, it included all of the existing primary health care workers in endemic communities who met the programme's prerequisite criteria of residency in that village and, where possible, literacy. The situation is similar in the samples of other national DEP for which we have data: Niger, Uganda, and Mali. When integration means using the resources which were procured for dracunculiasis eradication for other purposes, that is rarely justifiable, if at all. In my opinion when integration means turning over the active surveillance and stringent case containment which are required at the end of any eradication programme to an integrated health care system which is designed to control, not eradicate, diseases, precisely when the most intensive focus on interrupting transmission is needed, that is unwise. I believe the urgency which is unique to eradication programmes, and the demand for excellence in implementation which that urgency requires, cannot be integrated into broader primary health care or routine health services, even when those services are working well, much less when they are not. The rationale for the strategy of integrating control measures against dracunculiasis into other programmes appears sometimes to be motivated by a belief that the disease is not important enough to merit the intensive effort that is required to eradicate it, and by the wrong impression that control measures to eradicate dracunculiasis need to be "sustained". Aspects of these differences have been addressed recently in some publications (8-11), and I shall not repeat them here, but I would like to end this presentation by reviewing some of the indirect benefits of this eradication campaign. Benefits of the Eradication Programme Reducing the prevalence of dracunculiasis by almost 97% over the past decade is the most conspicuous achievement of the programme so far, even before eradication is fully achieved. The impact of that accomplishment on improved agricultural production alone is a major economic benefit and the World Bank, which considers an annual estimated rate of return (ERR) of greater than or equal to 10% as acceptable, has calculated an ERR of 29%, based on conservative assumptions of the duration of disability from dracunculiasis (12). Indirect contributions of the programme's success so far to improved school attendance, and to the nutrition of infants and the care of toddlers in endemic households, are no less real, despite being harder to quantify. Moreover, while realizing these accomplishments, DEP has accelerated and increased the provision of clean drinking-water by national and international agencies to thousands of endemic or formerly endemic communities, even after the Water and Sanitation Decade. It has also mobilized hundreds of communities to improve their own water supplies. In south-east Nigeria alone, for example, members of endemic villages have created more than 400 hand-dug wells in the past few years, in order to rid themselves of dracunculiasis. This is just one way that eradicating dracunculiasis has helped increase the self-reliance of some affected communities and generated ancillary benefits in the control of other waterborne diseases. The programme has also established community-based health education, village task forces, and surveillance by village volunteers in more than 15,000 remote villages (13). The very existence of some of those villages was previously unknown to other health workers. The nearly 6-month long "guinea-worm cease-fire" in Sudan in 1995 also provided opportunities to treat for the first time over 100,000 persons at risk of onchocerciasis, to vaccinate over 41,000 children against measles, 35,000 against poliomyelitis, and 22,000 against tuberculosis, and to distribute more than 35,000 doses of vitamin A and treat 9000 children with oral rehydration packets, in addition to jump-starting the DEP itself in that country (14). And despite our sometimes divergent views, dracunculiasis eradication has succeeded as much as it has because of a broad coalition of United Nations and bilateral assistance agencies, enormous private sector contributions by the DuPont Corporation, Precision Fabrics Group, American Home Products, nongovernmental organizations, national ministries, and political leaders, all of whom have contributed to help people in endemic communities to rid themselves of this parasite. People in these neglected communities need help. I have yet to visit an African village endemic for dracunculiasis or onchocerciasis which is suffering from too many visits by health care workers from different programmes, as some allege, requiring better integration or coordination of their health activities. The real problem is getting any health services to such communities. In the broad benefits it has provided and in its support of the public health staff and volunteers who are producing those benefits, one can assert with much justification that in addition to eradicating dracunculiasis, the Dracunculiasis Eradication Programme has done more to improve primary health care in endemic communities than many primary health care programmes. Primary health care was not developed in most of these communities before the DEP began, and not nearly enough is being done by health systems to build on that foundation and provide other needed services and support to the same communities once dracunculiasis is gone. I do not presume to represent the inhabitants of these neglected communities, but I do know that if I were in their place, I would prefer an excellent vertical programme to a mediocre integrated programme any day. Acknowledgements The assistance of Dr Eka Braide, Ms Nwando Diallo, Ms Renn Doyle, Ms Wanjira Mathai, Dr Ernesto Ruiz-Tiben and Dr James Zingeser in gathering or preparing some of the data for this paper is gratefully acknowledged. References
* The views expressed in this article are those of the author and may differ from those held by WHO. ** Associate Executive Director, The Carter Center, Atlanta, GA, USA. Figure 1 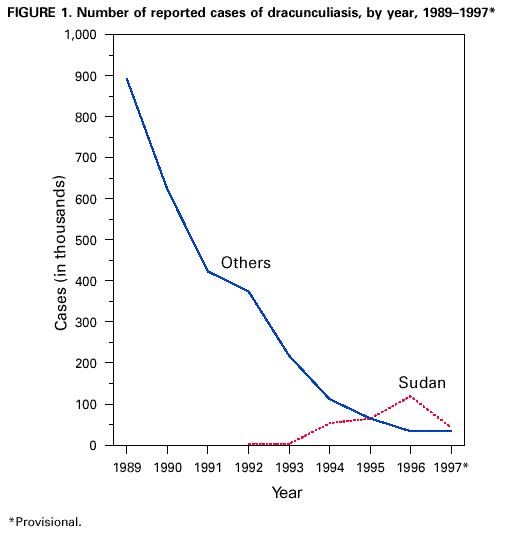 Return to top. Figure 2 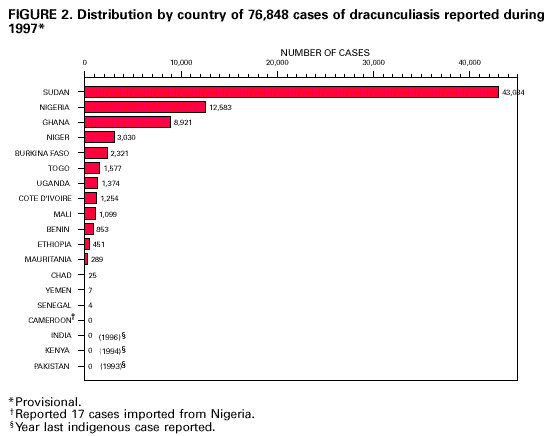 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 1/3/2000 |
|||||||||
This page last reviewed 5/2/01
|