Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail.
Imported Case of Marburg Hemorrhagic Fever --- Colorado, 2008
Marburg hemorrhagic fever (MHF) is a rare, viral hemorrhagic fever (VHF); the causative agent is an RNA virus in the family Filoviridae, and growing evidence demonstrates that fruit bats are the natural reservoir of Marburg virus (MARV) (1,2). On January 9, 2008, an infectious disease physician notified the Colorado Department of Public Health and Environment (CDPHE) of a case of unexplained febrile illness requiring hospitalization in a woman who had returned from travel in Uganda. Testing of early convalescent serum demonstrated no evidence of infection with agents that cause tropical febrile illnesses, including VHF. Six months later, in July 2008, the patient requested repeat testing after she learned of the death from MHF of a Dutch tourist who had visited the same bat-roosting cave as the patient, the Python Cave in Queen Elizabeth National Park, Uganda (3). The convalescent serologic testing revealed evidence of prior infection with MARV, and MARV RNA was detected in the archived early convalescent serum. A public health investigation did not identify illness consistent with secondary MHF transmission among her contacts, and no serologic evidence of infection was detected among the six tested of her eight tour companions. The patient might have acquired MARV infection through exposure to bat secretions or excretions while visiting the Python Cave. Travelers should be aware of the risk for acquiring MHF in caves or mines inhabited by bats in endemic areas in sub-Saharan Africa. Health-care providers should consider VHF among travelers returning from endemic areas who experience unexplained febrile illness.
Case Report
On January 1, 2008, the patient, a woman aged 44 years with no remarkable past medical history, returned to the United States from a 2-week safari in Uganda, where her activities included camping, white-water rafting, visiting local villages, and viewing wildlife. She had taken malaria prophylaxis with atovaquone-proguanil, as prescribed. On January 4, she experienced severe headache, chills, nausea, vomiting, and diarrhea (Figure). She self-treated for traveler's diarrhea with 2 doses of ciprofloxacin, and developed a diffuse rash. On January 6 and 7, she was seen as an outpatient, had laboratory testing performed, and was treated with antiemetics. A complete blood count on January 6 revealed an abnormally low white blood cell count of 900/µL (normal range: 4,500--10,500/µL). She returned to her primary-care physician's clinic on January 8, complaining of persistent diarrhea and abdominal pain, as well as worsening fatigue, generalized weakness, and confusion. On physical examination, she appeared pale and fatigued, and had decreased bowel sounds; the remainder of her examination was unremarkable. Laboratory results received on January 8 revealed hepatitis (aspartate aminotransaminase 9,660 U/dL [normal range: 15--41 U/L] and alanine aminotransferase 4,823 U/dL [normal range: 14--54 U/L]) and renal failure (creatinine 2.3 mg/dL [normal range: 0.7--1.2 mg/dL]). The patient was admitted to a community hospital for further management. The admission diagnosis was acute hepatitis, nausea, and vomiting of unknown etiology.
On admission, the patient was afebrile (temperature 96.2°F [35.7°C]). She was treated with intravenous fluids and was started on doxycycline for possible leptospirosis. Her hospital course was characterized by pancytopenia, coagulopathy, myositis, pancreatitis, and encephalopathy, all of which are complications that have been associated with MHF. She had no signs of gross hemorrhage other than vaginal bleeding attributed to menses. During her hospitalization, she underwent cholecystectomy for acalculous cholecystitis. Testing was negative for leptospirosis, viral hepatitis, malaria, arboviral infection, acute schistosomiasis, rickettsial infection, and VHFs (including Marburg and Ebola hemorrhagic fever) (Table). Early convalescent serum collected on January 14 (10 days after illness onset) was submitted to CDC for testing and demonstrated no evidence of MARV infection by virus isolation, antigen-detection enzyme-linked immunosorbent assay (ELISA), or anti-MARV immunoglobulin M (IgM) and IgG ELISA. The patient was discharged on January 19 and had a prolonged recovery over the following year because of persistent abdominal pain, fatigue, and "mental fog," but had no long-term sequelae such as chronic hepatitis or chronic renal disease. She received a blood transfusion for persistent anemia after she was discharged.
In July 2008, the patient requested repeat testing after she learned of the fatal case of MHF in a Dutch tourist who recently had visited the same cave she had visited in Uganda, the Python Cave. The Colorado patient had visited the cave on December 25, 2007, 10 days before onset of her initial symptoms. Serum collected on July 15 tested positive for anti-MARV IgG by ELISA, prompting additional testing of the archived day 10 serum. Traditional reverse-transcriptase polymerase chain reaction (RT-PCR) was negative, and real-time (Taqman) RT-PCR was equivocal; however, nested RT-PCR* confirmed the presence of MARV RNA fragments in the day 10 sample.
Public Health Response
On January 22, 2009, CDC notified the World Health Organization and Uganda Ministry of Health of the imported MHF case. The Python Cave had already been closed to visitors in July 2008, during the response to the Dutch MHF case. CDPHE and CDC conducted a public health investigation during January--February 2009. Interviews were conducted with the patient and her spouse, the patient's medical records were reviewed, and a retrospective contact investigation was conducted to identify possible secondary transmission. A contact was defined as a person who had physical contact with the patient, her body fluids, or contaminated materials or was in the same room as the patient during her acute illness (January 4--19, 2008). Contacts included health-care workers (including health-care providers, housekeeping staff, and hospital laboratory staff), commercial laboratory staff, and social contacts.
To limit the effect of recall bias and to identify secondary cases of MHF, a contact-tracing protocol (4) was modified for retrospective use to identify contacts who had a high-risk exposure to the patient's body fluids (through splash, percutaneous, or nonintact skin exposure), or prolonged absenteeism of ≥7 days as indicated by review of health and payroll records. The contact investigation identified approximately 260 contacts: 220 health-care workers, approximately 30 commercial laboratory workers from five laboratories, and 10 social contacts. No high-risk exposure or severe febrile illness was identified.
The patient and her spouse reported spending approximately 15--20 minutes in the cave and recalled seeing bats flying overhead. Neither remembered her having contact with a bat or sustaining an injury in the cave. However, the patient reported touching guano-covered rocks while climbing into the cave and surmised that she might have covered her mouth and nose with her hands once inside because of the unpleasant smell.
CDC, with assistance from public health agencies in Illinois, Uganda, Belgium, and the United Kingdom, conducted an investigation of the eight tour companions who accompanied the patient when she visited the Python Cave. During February--July 2009, participants were interviewed using a standardized questionnaire by telephone or e-mail and were offered serologic testing by anti-MARV IgG ELISA. Questionnaires were completed for all eight tour companions. All eight reported having entered the cave (at least under the cave ceiling), and six reported climbing over a crop of boulders further inside as the patient had done; however, none reported direct contact with bats or bat guano/urine. Serum samples were provided by six of the tour companions; none had evidence of prior MARV infection by anti-MARV IgG.
Reported by: N Fujita, MD, Western Infectious Disease Consultants, Wheat Ridge; A Miller, Exempla Lutheran Medical Center, Wheat Ridge; G Miller, DVM, Jefferson County Public Health; K Gershman, MD, Colorado Dept of Public Health and Environment. Special Pathogens Br, Div of Viral and Rickettsial Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases; N Gallagher, N Marano, DVM, Div of Global Migration and Quarantine, National Center for Prevention, Detection, and Control of Infectious Diseases; C Hale, DVM, E Jentes, PhD, EIS officers, CDC.
Editorial Note:
Before the case described in this report, the only human cases of VHF imported into the United States were single cases of Lassa fever (an arenaviral hemorrhagic fever) in Chicago, Illinois, in 1989 (5) and in Trenton, New Jersey, in 2004 (4). No previous cases of imported filovirus (MARV or Ebola virus) infections have been reported in the United States, making this the first imported case of a filoviral hemorrhagic fever in the United States.
The patient described in this report was first diagnosed by convalescent serology because initial testing of the day 10 sample was negative by virus isolation, antigen-detection, and IgM and IgG ELISA. After the Dutch patient was diagnosed with MHF, retesting of the archived specimen with more sensitive molecular methods was performed, including a nested RT-PCR assay that detected viral RNA. This, along with the positive convalescent serology and compatible clinical course, confirmed the diagnosis. To obtain a rapid diagnosis during the acute illness, patients with suspected VHF should have paired acute blood specimens (ideally collected during days 0--4 and days 4--9 of the acute illness) tested at a World Reference Laboratory (e.g., CDC) with biosafety level 4 capability using multiple methods as appropriate for the timing of the sample, including virus isolation, RT-PCR, and IgM and IgG ELISA. Because the incubation period for MARV is 2--21 days, daily contact tracing is recommended to contain outbreaks. This involves following all contacts of patients suspected of having MHF, and isolating and testing those that experience fever within 21 days after their last contact.
Other sporadic cases of MHF have been reported outside of Africa: two laboratory-acquired cases in Russia and two cases imported from endemic areas (3,6). These imported cases occurred in a patient hospitalized in South Africa who likely acquired the disease while camping in Zimbabwe in 1975 (6) and the second in the previously described Dutch patient hospitalized in the Netherlands who died of MHF after visiting the Python Cave in Uganda in 2008 (3). Case-fatality rates of 83%--90% have been reported for widespread outbreaks of MHF in Africa (1,7).
Virologic and serologic evidence of MARV infection has been documented among cave-dwelling bats, particularly the Egyptian fruit bat Rousettus aegyptiacus (2); this evidence has implicated bats as the likely natural reservoir for MARV. R. aegyptiacus bats have a wide range covering most of Africa, indicating that risk for zoonotic infection might exist beyond areas with previously documented cases. The precise route of MARV transmission from the putative bat reservoir to humans has not been determined and might include direct or indirect exposure to bat excretions and secretions. MHF outbreaks have resulted from exposure to caves or mines inhabited by bats (1,8) and subsequent human-to-human transmission through direct contact with infectious body fluids and contaminated materials, primarily affecting caregivers and health-care workers (8,9). Isolation of suspected patients and implementation of droplet and contact precautions are recommended to prevent person-to-person spread.†
Although the Python Cave is closed and no additional MHF cases have been reported, travelers should be aware of the risk for acquiring MHF in endemic areas in Africa and should avoid entering caves or mines inhabited by bats in these areas (10). Health-care providers should have a low threshold of suspicion for VHF among travelers returning from endemic areas, promptly implement appropriate infection control measures, and rapidly report suspected cases. Suspected cases of VHF are nationally notifiable and should be reported immediately to local and state health departments and to CDC's Special Pathogens Branch at 404-639-1115 (770-488-7100 after hours) to obtain guidance on testing, management, and response. Additional information regarding Marburg hemorrhagic fever,§ travelers' health,¶ and VHF infection-control guidelines** are available online.
Acknowledgments
This report is based, in part, on contributions by J Desjardin, MD, Western Infectious Disease Consultants, Wheat Ridge, Colorado; C Austin, Illinois Dept of Health; M Sabbe, MD, S Quoilin, MD, D Reynders, MD, Scientific Institute of Public Health, Brussels, Belgium; A Walsh, MSc, Y Chow, MBBS, D Morgan, MD, Health Protection Agency, London, United Kingdom; S Balinandi, MSc, R Downing, PhD, CDC-Uganda; J Lutwama, PhD, Uganda Virus Research Institute.
References
- Swanepoel R, Smit S, Rollin P, et al. Studies of reservoir hosts for Marburg virus. Emerg Infect Dis 2007;13:1847--51.
- Towner JS, Amman BR, Sealy TK, et al. Isolation of genetically diverse Marburg viruses from Egyptian fruit bats. PLoS Pathog 2009;5:e1000536.
- Timen A, Koopmans M, Vossen A, et al. Response to imported case of Marburg hemorrhagic fever, the Netherlands. Emerg Infect Dis 2009;15:1171--5.
- CDC. Imported Lassa fever---New Jersey, 2004. MMWR 2004;53:894--7.
- Holmes GP, McCormick JB, Trock SC, et al. Lassa fever in the United States: Investigation of a case and new guidelines for management. N Engl J Med 1990;323:1120--3.
- Slenczka W, Klenk HD. Forty years of Marburg virus. J Infect Dis 2007;196(Suppl 2):S131--5.
- Towner JS, Khristova ML, Sealy TK, et al. Marburg virus genomics and association with a large hemorrhagic fever outbreak in Angola. J Virol 2006;80:6497--516.
- Bausch DG, Borchert M, Grein T, et al. Risk factors for Marburg hemorrhagic fever, Democratic Republic of the Congo. Emerg Infect Dis 2003;9:1531--7.
- Borchert M, Mulangu S, Lefèvre P, et al. Use of protective gear and the occurrence of occupational Marburg hemorrhagic fever in health workers from Watsa Health Zone, Democratic Republic of the Congo. J Infect Dis 2007;196(Suppl 2):S168--75.
- CDC. Viral hemorrhagic fevers. In: CDC health information for international travel 2010. Atlanta, GA: US Department of Health and Human Services, Public Health Service; 2009:406--9.
* Nested RT-PCR is more sensitive and specific than traditional RT-PCR. A portion of the product produced from the first round of amplification is used in the second round of amplification along with a different set of primers.
† Based on CDC's Interim Guidance for Managing Patients with Suspected Viral Hemorrhagic Fever in U.S. Hospitals, available at http://www.cdc.gov/ncidod/dhqp/bp_vhf_interimguidance.html.
§ Available at http://www.cdc.gov/ncidod/dvrd/spb/mnpages/dispages/marburg.htm.
¶ Available at http://wwwn.cdc.gov/travel.
** Available at http://www.cdc.gov/ncidod/dhqp/bp_vhf_interimguidance.html.
|
What is already known on this topic? Marburg hemorrhagic fever (MHF) is a rare viral hemorrhagic fever caused by Marburg virus (a filovirus in the same family as Ebola virus), which is endemic in tropical areas of Africa and likely is maintained in nature by cave-dwelling bats. What is added by this report? The case described in this report, the first imported case of a filoviral hemorrhagic fever in the United States, adds further support to the epidemiologic link between MHF and exposure to caves inhabited by bats in Africa. What are the implications for public health practice? Health-care providers should advise travelers to endemic areas of Africa to avoid entering caves inhabited by bats, should consider the diagnosis of viral hemorrhagic fever among severely ill travelers returning from endemic areas, and should rapidly report, isolate, and test patients with suspected cases. |
FIGURE. Timeline of key events in the treatment and diagnosis of an imported case of Marburg hemorrhagic fever (MHF) --- Colorado, December 2007--January 2009
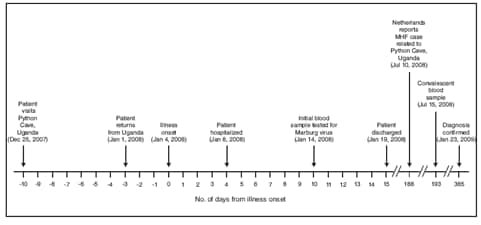
Alternate Text: The figure above shows the timeline of key events in the treatment and diagnosis of an imported case of Marburg hemorrhagic fever in Colorado from December 25, 2007 through January 23, 2008.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the U.S. Department of
Health and Human Services. |
All MMWR HTML versions of articles are electronic conversions from typeset documents. This conversion might result in character translation or format errors in the HTML version. Users are referred to the electronic PDF version (http://www.cdc.gov/mmwr) and/or the original MMWR paper copy for printable versions of official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices.
**Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Date last reviewed: 12/17/2009


