 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update: Influenza Activity -- United States, 1997-98 SeasonIn collaboration with the World Health Organization (WHO), its collaborating laboratories, and state and local health departments, CDC conducts surveillance to monitor influenza activity and to detect antigenic changes in the circulating strains of influenza viruses. This report summarizes influenza surveillance in the United States from September 1 through December 12, 1997, which indicates that influenza activity is at typical levels for this time of year and that influenza A(H3N2) viruses have been most commonly isolated. From September 1 through December 12, influenza A viruses were reported from 25 states and the District of Columbia, and influenza B viruses were reported from three states (Figure_1). From September 28 through December 6, a total of 68 of 11,705 respiratory specimens tested by WHO collaborating laboratories in the United States were positive for influenza viruses. Of these, 66 (97%) were influenza type A, and two (3%) were influenza type B. All influenza A viruses that were subtyped were influenza A(H3N2). Of the 22 influenza A(H3N2) viruses antigenically characterized by CDC, 16 were A/Nanchang/933/95-like, the H3N2 component of the 1997-98 influenza vaccine, and six were similar to A/Sydney/05/97, a related but antigenically distinguishable strain that was first detected in Australia and New Zealand during June 1997 (1). One A/Sydney/05/97-like virus was isolated in the continental United States; this isolate was recently cultured from an infant in New York. For the week ending December 6, state and territorial epidemiologists reported regional activity in one state and sporadic activity in 21 states, the District of Columbia, and Puerto Rico. * The percentage of patient visits to sentinel physicians for influenza-like illness remained within baseline levels (0-3%) during October through early December, and the percentage of deaths attributed to pneumonia and influenza as reported by the vital statistics offices of 122 cities has not exceeded the epidemic threshold this season. Reported by: Participating state and territorial epidemiologists and state public health laboratory directors. World Health Organization collaborating laboratories. WHO Collaborating Center for Surveillance, Epidemiology, and Control of Influenza, Influenza Br, Div of Viral and Rickettsial Disease, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: The level of influenza activity in the United States this year has been typical for October through early December. Although the optimal time for influenza vaccination is October through mid-November, health-care providers should continue to offer influenza vaccine up to and even after influenza activity has been detected in the community, particularly to those persons at high risk for influenza-related complications (2). When influenza vaccine is administered after local outbreaks of influenza type A have been reported in a community, short-term prophylaxis with amantadine or rimantadine can be offered. Although these drugs are useful for treatment or prophylaxis for influenza type A infection, they are not effective against influenza type B. Most H3N2 viruses antigenically characterized this season have been similar to the vaccine strain A/Nanchang/933/95(H3N2). The extent to which the antigenic variant A/Sydney/05/97 will circulate in the United States this season cannot be predicted. Vaccine effectiveness is dependent, in part, on the match between the vaccine and circulating strains; wider circulation of the variant might be associated with suboptimal vaccine protection (3-5). Even when the match between circulating strains and the vaccine strain is good, outbreaks of influenza can still occur among vaccinated persons. In settings, such as nursing homes, that house persons at high risk for influenza-related complications, contingency plans for rapid diagnostic testing for influenza type A viruses can help detect outbreaks early and guide the usage of antiviral drugs for prophylaxis and treatment. Throughout the season, influenza surveillance data are updated weekly and are available through CDC's fax information system, telephone (888) 232-3299 by requesting document number 361100 and entering the telephone number to which the document should be transmitted, or through CDC's Influenza Branch, Division of Viral and Rickettsial Diseases, National Center for Infectious Diseases, World-Wide Web site http://www.cdc.gov/ncidod/diseases/flu/weekly.htm. References
* Levels of activity are 1) no activity; 2) sporadic -- sporadically occurring influenza-like illness (ILI) or culture-confirmed influenza, with no outbreaks detected; 3) regional -- outbreaks of ILI or culture-confirmed influenza in counties having a combined population of less than 50% of the state's total population; and 4) widespread -- outbreaks of ILI or culture-confirmed influenza in counties having a combined population of greater than or equal to 50% of the state's total population. Figure_1 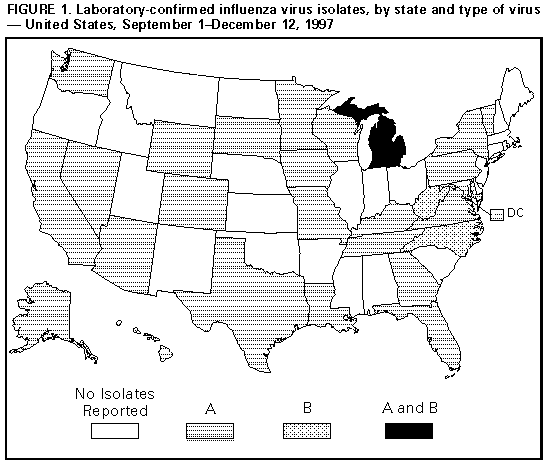 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|