 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Update -- Progress Toward Poliomyelitis Eradication -- Socialist Republic of Vietnam, 1993-1994In 1988, the Western Pacific Region (WPR) of the World Health Organization (WHO) adopted a resolution to eradicate poliomyelitis from the region by the end of 1995. In 1993, the Socialist Republic of Vietnam (1993 population: 70.9 million) accounted for 452 (40%) of the 1147 cases of confirmed polio reported to WPR-WHO. Efforts to eradicate polio in Vietnam were initiated in 1991 using supplementary vaccination activities with oral poliovirus vaccine (OPV). National Immunization Days (NIDs) * were first conducted during November-December 1993. This report updates these efforts and describes the impact of the first NIDs in 1993 (1). National Immunization Days The first NIDs were conducted during November 13-15 and December 18-20, 1993, targeting children aged less than 5 years. Two doses of OPV were administered to each of 9.7 million children. An estimated 10%-15% of vaccinated children were aged greater than or equal to 5 years; coverage of children aged less than 5 years with two doses of OPV was 83%-88%. NIDs were repeated during November 12-14 and December 17-19, 1994; two doses of OPV were administered to each of 10.0 million children. An estimated 5%-10% of vaccinated children were aged greater than or equal to 5 years; coverage of children aged less than 5 years with two doses of OPV was 89%-94%. The third NIDs in Vietnam are scheduled for November 11-13 and December 16-18, 1995. Surveillance for Polio A surveillance system implemented in Vietnam in 1991 defines a suspected case of polio as acute flaccid paralysis (AFP) in a person aged less than 15 years. Two stool specimens are collected from each person suspected to have polio at an interval of 24-48 hours to detect the presence of wild poliovirus. Each suspected case is investigated after 60 days to assess for residual paralysis. Of 607 persons with AFP reported in 1993, at least one stool specimen was collected for 381 (63%), and polio was confirmed ** in 452 (74%); wild polioviruses were isolated from 152 persons in 74 (13%) of 560 districts, including 21 in the northern region (Red River Delta), five in the central region, two in the Highlands region, and 46 in the southern region (Mekong Delta). The last person with wild poliovirus isolated in the northern region had onset on November 8, 1993. Of 152 persons with wild poliovirus isolated, 50 (33%) were children aged 0-23 months, and 127 (84%) were children aged less than 5 years. Of 97 persons aged 1-4 years from whom wild poliovirus was isolated and for whom vaccination status was known, 63 (65%) had received no previous dose or one dose of OPV. Of 353 persons with AFP reported in 1994, at least one stool specimen was collected for 262 (74%), two stool specimens were collected for 207 (59%), and one stool specimen was collected within 0-14 days of onset of paralysis for 228 (65%); polio was confirmed in 124 (35%) Figure_1. Wild polioviruses were isolated from 31 persons in 25 districts, including one in the Highlands region and 24 in the southern region (Mekong Delta). The last person with wild poliovirus isolated in the southern region had onset on December 14, 1994. No wild poliovirus was isolated from 164 AFP patients in the northern region and from 22 AFP patients in the central region, of which 132 (80%) and 11 (50%), respectively, had at least one stool specimen collected. A total of 229 AFP cases were determined not to be polio, or 0.8 AFP cases per 100,000 children aged less than 15 years (a reference rate of greater than or equal to 1.0 per 100,000 children aged less than 15 years is used to define a sensitive AFP surveillance system). Reported by: Dang D Trach, MD, Tran V Tien, MD, Do S Hien, MD, Phan VD Hang, PhD, Nguyen V Cuong, MD, Nguyen T Yen, MD, Thanh K Dung, MD, Nguyen TH Thanh, PhD, Pham TN Oanh, MD, Expanded Program on Immunization, National Institute of Hygiene and Epidemiology, Hanoi; Le D Hinh, MD, Bach Mai Hospital, Hanoi; Nguyen V Man, MD, Poliomyelitis Vaccine Research and Production Center, Hanoi; Doan T Tam, MD, National Center for Vaccine Quality Control, Hanoi; Ha B Khiem, MD, Pham K Sac, MD, Nguyen TT Thuy, MD, Van TT Binh, MD, Nguyen M Phuong, MD, Vu Q Ai, MD, Phan V Tu, MD, Nguyen T Long, MD, Pasteur Institute, Ho Chi Minh City; Vo C Khanh, MD, Center for Tropical Diseases, Ho Chi Minh City; Nguyen TT Tram, MD, Pasteur Institute, Nha Trang; Nguyen A Phuong, MD, Institute of Hygiene and Epidemiology, Ban Me Thuot, Socialist Republic of Vietnam. Expanded Program on Immunization Unit, Western Pacific Regional Office, World Health Organization, Manila, Philippines. Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases; Polio Eradication Activity, National Immunization Program, CDC. Editorial NoteEditorial Note: The findings in this report suggest that the first NIDs in Vietnam in 1993 were highly effective in reducing circulating wild poliovirus to low levels, particularly in the northern and central regions of Vietnam. Before the NIDs, wild poliovirus was documented in 74 (13%) of 560 districts. Since implementation of the first NIDs, wild poliovirus has been detected in 25 (4%) districts, including 24 in the southern Mekong Delta region in which the incidence has been the highest. Confirmed cases declined dramatically in all age groups, including among children aged greater than or equal to 5 years not targeted during NIDs, indicating that supplemental vaccination with OPV in children aged less than 5 years may be sufficient to interrupt wild poliovirus circulation in older age groups. Reported cases of polio and the number of cases of wild poliovirus have declined despite improvement in the sensitivity of surveillance. The progress toward eradication of polio in Vietnam reflects the collaborative efforts of many organizations, including WHO, Rotary International, United Nations Children's Fund (UNICEF), and government agencies including Japan International Cooperation Agency (JICA), Japan National Institutes of Health, the Australia Agency for International Development (AusAID), CDC, and the government of Luxembourg. Continued progress toward the goal will require successful implementation of at least five strategies: 1) improving the reporting of AFP patients to achieve a rate of greater than or equal to 1.0 per 100,000 children aged less than 15 years in every province (2); 2) increasing to 80% in every province the percentage of AFP patients for whom two stool specimens are obtained within 0-14 days of onset of paralysis; 3) intensifying surveillance and supplemental vaccination in areas with documented or suspected circulation of wild poliovirus (i.e., the Mekong Delta region); 4) using a more specific surveillance case definition based on virologic confirmation of AFP cases; and 5) preventing reimportation of wild poliovirus into Vietnam from neighboring polio-endemic countries (the first NIDs in Cambodia were conducted during February-March 1995). The effectiveness of these strategies to rapidly reduce the circulation of wild poliovirus is indicated by the successful eradication of wild poliovirus in the Americas (3), the experience in China (4), and the current progress in Vietnam. References
* Mass campaigns over a short period (days to weeks) in which two doses of OPV are administered to all children in the target group regardless of prior vaccination history, with an interval of 4-6 weeks between doses. ** A confirmed case of polio is defined as AFP and at least one of the following: 1) laboratory-confirmed wild poliovirus infection, 2) residual paralysis at 60 days, 3) death, or 4) lost to follow-up investigation at 60 days. Cases in 1990-1991 were reported by clinicians as confirmed polio before the standard WHO criteria were in use. Figure_1 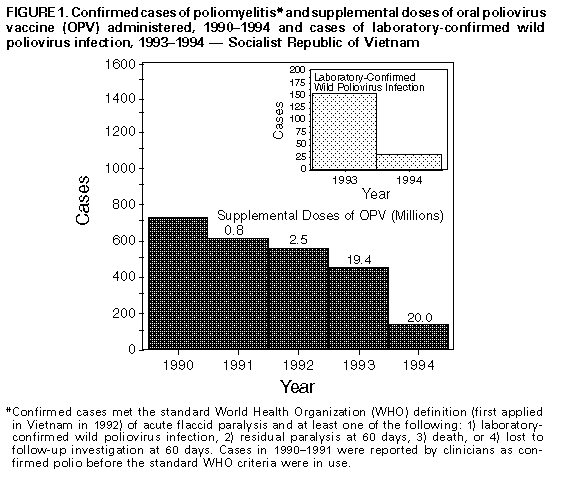 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|