 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Current Trends Update: Respiratory Syncytial Virus Activity -- United States, 1994-95 SeasonRespiratory syncytial virus (RSV), a common cause of winter outbreaks of acute respiratory disease, causes an estimated 90,000 hospitalizations and 4500 deaths each year from lower respiratory tract disease in both infants and young children in the United States (1). Outbreaks occur annually throughout the United States, and community activity usually peaks within 1 month of the national peak (2). RSV activity in the United States is monitored by the National Respiratory and Enteric Virus Surveillance System (NREVSS), a voluntary, laboratory-based system. This report presents provisional surveillance results from the NREVSS for RSV during July 2-December 9, 1994, and summarizes trends in RSV from July 1, 1990, through July 1, 1994. Since July 1, 1990, a total of 105 hospital-based and public health laboratories in 47 states have participated in the NREVSS and have reported weekly to CDC the number of specimens tested for RSV by the antigen detection and virus isolation methods and the number of positive results. Widespread RSV activity is defined by the NREVSS as the first of 2 consecutive weeks when at least half of participating laboratories report any RSV detections. This definition generally indicates a mean percentage of specimens positive by antigen detection in excess of 10%. During the previous four seasons, from July 1, 1990, through July 1, 1994, onset of widespread RSV activity began in November and continued an average of 24 weeks until April or mid-May Figure_1. The peak in activity occurred each year from mid-January through mid-February. For the current reporting period (July 2-December 9, 1994), 85 laboratories in 43 states reported results of testing for RSV. Since November 12, more than half of the participating laboratories reported detections of RSV on a weekly basis, indicating the onset of RSV activity for the 1994-95 season. Reported by: National Respiratory and Enteric Virus Surveillance System laboratories. Respiratory and Enteric Viruses Br, Div of Viral and Rickettsial Diseases, National Center for Infectious Diseases, CDC. Editorial NoteEditorial Note: During the RSV season, health-care providers should consider RSV as a cause of acute respiratory disease in both children and adults. Most severe manifestations of infection with RSV (e.g., pneumonia and bronchiolitis) occur in infants aged 2-6 months; however, children of any age with underlying cardiac or pulmonary disease or who are immunocompromised are at risk for serious complications from this infection. Because natural infection with RSV provides limited protective immunity, RSV may cause repeated symptomatic infections throughout life. In adults, RSV usually causes upper respiratory tract manifestations but may cause lower respiratory tract disease -- especially in the elderly and in immunocompromised persons. Outbreaks among immunocompromised persons can result in high death rates. RSV is a common, but preventable, cause of nosocomially acquired infection; the risk for nosocomial transmission is increased during community outbreaks. Sources for nosocomially acquired infection include infected patients, staff, visitors, or contaminated fomites. Nosocomial outbreaks or transmission of RSV can be controlled with strict attention to contact-isolation procedures (3). In addition, chemotherapy with ribavirin is indicated for some patients (e.g., those at high risk for severe complications or those who are seriously ill with this infection) (4). Prophylaxis with intravenous RSV immunoglobulin for high-risk patients is being evaluated (5). Vaccines for RSV are being developed, and some are being evaluated in clinical trials; however, none have been proven safe and efficacious (6). References
Figure_1 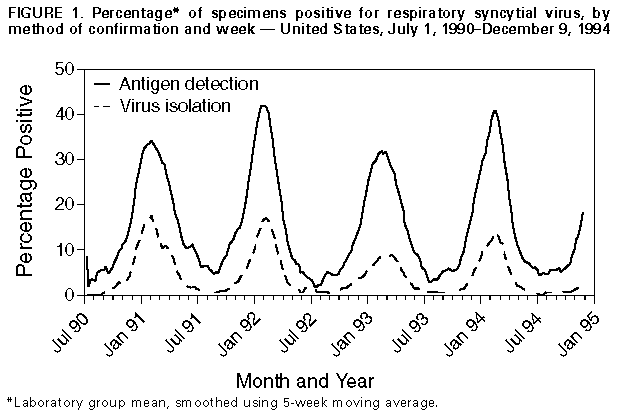 Return to top. Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 09/19/98 |
|||||||||
This page last reviewed 5/2/01
|