 |
|
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
|
||||
| ||||||||||
|
|
|
|
|
Persons using assistive technology might not be able to fully access information in this file. For assistance, please send e-mail to: mmwrq@cdc.gov. Type 508 Accommodation and the title of the report in the subject line of e-mail. Experiences with Obtaining Influenza Vaccination Among Persons in Priority Groups During a Vaccine Shortage --- United States, October--November, 2004After the announcement that the supply of inactivated influenza vaccine available to the U.S. public for the 2004--05 influenza season would be reduced by approximately one half, the Advisory Committee on Immunization Practices (ACIP) recommended that the remaining vaccine supply should be reserved for 1) certain groups of persons at high risk for serious health problems from influenza, 2) health-care workers involved in direct patient care, and 3) close contacts of children aged <6 months (1). To determine what proportion of persons at increased risk for influenza complications had been vaccinated as of the day of the survey, what proportion sought vaccination but did not receive it because of the shortage, and what factors might be dissuading persons at high risk from seeking influenza vaccination, Harvard School of Public Health (HSPH), in collaboration with International Communications Research, conducted a national survey. This report summarizes the results of that survey, which indicated that approximately 63% of persons aged >65 years and 46% of chronically ill adults who tried to get the influenza vaccine were able to do so. More than half of adults at high risk did not try to get the influenza vaccine. Because available supplies of inactivated influenza vaccine are targeted to high-risk groups, persons in these groups should continue to pursue vaccination. HSPH provides CDC with technical assistance for public health communication by monitoring the response of the general public to public health threats. National polling on what the public knows, believes, and experiences in regard to seeking and receiving influenza vaccination during a national vaccine shortage is the basis of the data presented in this report. During October 29--November 9, 2004, telephone interviews were conducted to assess experiences of respondents with obtaining the influenza vaccine. The survey was conducted by International Communications Research as part of an omnibus survey. The omnibus survey is a national, biweekly telephone survey that can include questions on several topics; however, because of the length of the questionnaire, the omnibus survey regarding influenza vaccination only included the HSPH questions. Respondents were asked 1) if they tried to get the influenza vaccine during the preceding 3 months, 2) if so, whether they were able to get the vaccine, and 3) whether they experienced any problems while trying to get the vaccine. Respondents who did not try to get the vaccine were asked why they did not. Respondents were also asked about their willingness to receive an imported influenza vaccine not licensed for general use in the United States. Parents of children aged 6--23 months were asked these questions about their children in that age group. The questionnaire was administered to adults aged >18 years who were selected by using a fully replicated, stratified, single-stage, random-digit--dialing sample of households nationally*. Within each household, an adult respondent was randomly selected by asking for the adult with the most recent birthday. A total of 1,227 adults completed interviews. This group included an oversample of parents with children aged 6--23 months. A total of 249 interviews were completed with this latter group. Parents were asked vaccine-related questions about each of their children in the age group. The data analysis targeted three groups at high risk included among those prioritized by ACIP for influenza vaccination in 2004: 1) persons aged >65 years, 2) persons aged 18--64 years with underlying chronic medical conditions, and 3) children aged 6--23 months. The data were weighted to account for the disproportionate probability of household selection attributable to multiple telephone lines and the probability associated with the random selection of an individual household member. Following the application of the above weight, the sample was post-stratified and balanced by age, sex, race/ethnicity, education, region, census division, and metropolitan status to be nationally representative. Statistical software was used to calculate standard errors for weighted data. Confidence intervals (CIs) also were calculated. Adults in Priority GroupsAmong adult respondents, 242 (19%) were aged >65 years; 306 (25%) had been told by a doctor that they had one of the following health conditions: heart or lung disease, asthma, kidney disease, diabetes, or a disease that causes decreased immunity (e.g., cancer or HIV/AIDS). For this analysis, these groups were combined and referenced as adults at high risk (n = 427), unless otherwise noted. Among adults aged >65 years, 119 (49%) tried to get the influenza vaccine during the preceding 3 months. Among those in this age group who tried to get the vaccine, 75 (63%) were able to get the vaccine, and 44 (37%) were unable to do so. A total of 113 (37%) adults with a chronic illness tried to get the vaccine; among those who tried to get the vaccine, 52 (46%) were able to get it, whereas 61 (54%) reported being unable to do so (Table 1). Respondents were asked to rate problems as either major problems they experienced when trying to get the vaccine, minor problems, or not problems at all (Table 2). The leading problems experienced by the 81 adults at high risk who tried and could not get the vaccine included the following: 1) no vaccine was available when they tried to get it (55 [68%] cited this as a major problem) and 2) finding a place where they could get the vaccine was difficult (41 [50%]). Among the 427 adults at high risk as defined above, 257 (60%) (CI = 54%--66%) reported that they did not try to get the influenza vaccine during the preceding 3 months. Awareness of the influenza vaccine shortage was an important reason cited for not trying to get the vaccine: 82 of these 257 (32%) (CI = 24%--40%) said either that they were waiting until more vaccine was available or that they believed that, because of shortages, they could not get the vaccine. Other major reasons included 1) believing that they were not at high risk for getting a serious case of influenza (53 [21%]; CI = 14%--27%), 2) not believing that the vaccine would be effective in preventing them from getting influenza (45 [18%]; CI = 11%--25%), and 3) concerns that they could get influenza from the vaccine (46 [18%]; CI = 12%--25%). Children Aged 6--23 MonthsOf parents with children aged 6--23 months, 125 (50%) (CI = 39%--59%) tried to get the vaccine for their child; 95 (76%) of those parents who tried to get the vaccine for their child reported that they were able to get the influenza vaccine, and 30 (24%) reported that they were unable to do so (Table 1). Few problems were reported by parents who tried to get the vaccine. A total of 14 (11%) (CI = 1%--17%) parents who tried to get the vaccine for their child reported problems, including 1) difficulty finding vaccine, 2) inconvenient times, and 3) a health-care provider advising against their child receiving vaccine because of the shortages or for a medical reason. For children aged 6--23 months, the leading reasons for not trying to get inactivated influenza vaccine reported by parents were 1) not believing their children were at risk for a serious case of influenza (26 [21%]; CI = 10%--37%); 2) concern about the side effects (24 [19%]; CI = 6%--32%); 3) being told by a health-care provider that the child should not get the vaccine because of the shortages and because the child was not at high risk for having a serious case of influenza (22 [18%]; CI = 7%--34%); and 4) not believing that the influenza vaccine was effective (16 [13%]; CI = 4%--22%). Importation of Influenza Vaccine Not Licensed by FDATo ease the vaccine shortage in the United States, the U.S. government has announced its intention to import from Germany influenza vaccine not licensed by the Food and Drug Administration (FDA). The vaccine, Fluarix™ (GlaxoSmithKline, Dresden, Germany), although fully licensed for use in Germany, is not approved for general use in the United States and is therefore considered to be investigational. Respondents were asked if they would be willing to take the vaccine after being told that the vaccine was investigational. Fifty-six percent (CI = 49%--63%) of adults at high risk said they would be willing to receive this vaccine if no other vaccine were available. U.S. persons who elect to receive investigational vaccines are required to sign a form. With this requirement imposed, willingness to take the vaccine decreased to 40% (CI = 34%--46%) among adults at high risk. Reported by: RJ Blendon, ScD, CM DesRoches, DrPH, JM Benson, MA, KJ Weldon, Harvard School of Public Health, Boston, Massachusetts. Editorial Note:The findings in this report suggest that, during the current vaccine shortage, approximately 63% of persons aged >65 years and 46% of chronically ill adults who tried to get the influenza vaccine were able to do so. However, more than half of adults at high risk did not try to get the influenza vaccine. For many of these respondents, this was because of perceived shortages, underscoring the need to continue to encourage these groups to pursue vaccination. Efforts to vaccinate these groups should include measures to educate them about the severity of influenza and the effectiveness of the vaccine and address unwarranted fears of getting influenza from the vaccine. Finally, the reluctance expressed by adults in priority groups about receiving imported influenza vaccine not licensed by FDA suggests the need for educational efforts to provide reassurance that this vaccine is approved for use in Germany by government agencies similar to the FDA. In 2004, for the first time, ACIP recommended that children aged 6--23 months be vaccinated. The findings in this report suggest that parents of children in this age group who tried to get the vaccine for their children experienced fewer difficulties in getting the vaccine than persons aged >65 years or those with chronic illnesses. The findings in this report are subject to at least two limitations. First, because the study was conducted as part of an omnibus survey, the data are not collected in a way that allows for the calculation of the response rate. However, studies have indicated that when the results from a survey with a long field period and high response rate are compared with a survey with a field time that is similar to the HSPH survey, few statistically significant differences are observed between responses from the two surveys when the data are statistically reweighted (3--6). Second, the survey sample included only noninstitutionalized persons. Nursing home residents, who are excluded from the sample, might receive the influenza vaccine at a different rate than those in the study sample. The results of the HSPH survey differ from those of the Behavioral Risk Factor Surveillance System (BRFSS) survey, also published in this issue (2). Important differences in survey methodologies might contribute to the differences in results. The primary differences are that 1) the surveys were conducted during different periods (i.e., October 29--November 9 for HSPH and December 1--11 for BRFSS); 2) somewhat different questions were asked; and 3) the HSPH data came from a single, national sample, but BRFSS data were collected individually by 48 states and the District of Columbia. Despite these differences, both surveys demonstrate a substantial need for the influenza vaccine that has not been met. Assuming that an adequate vaccine supply will be available for persons in priority groups this season, health-care providers should continue to emphasize 1) the need for these groups to get vaccinated this season and 2) the availability of vaccine allowing all persons in these groups to get vaccinated. Influenza vaccine should continue to be directed to areas most affected by the shortage. References
* Similar questions were asked in the Behavioral Risk Factor Surveillance System survey reported in this issue of MMWR (2).
Table 1 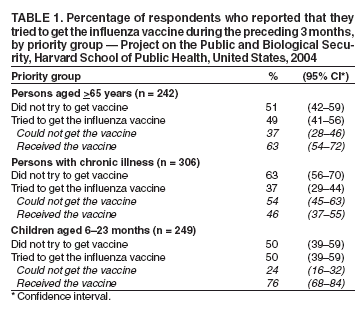 Return to top. Table 2 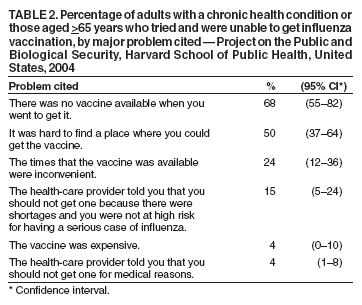 Return to top.
Disclaimer All MMWR HTML versions of articles are electronic conversions from ASCII text into HTML. This conversion may have resulted in character translation or format errors in the HTML version. Users should not rely on this HTML document, but are referred to the electronic PDF version and/or the original MMWR paper copy for the official text, figures, and tables. An original paper copy of this issue can be obtained from the Superintendent of Documents, U.S. Government Printing Office (GPO), Washington, DC 20402-9371; telephone: (202) 512-1800. Contact GPO for current prices. **Questions or messages regarding errors in formatting should be addressed to mmwrq@cdc.gov.Page converted: 12/16/2004 |
|||||||||
This page last reviewed 12/16/2004
|