Multistate Investigation of Suspected Infections Following Steroid Injections
- Reported Cases: 26
- States reporting cases: 4
- Recall: Yesexternal icon
Initial Announcement
May 30, 2013
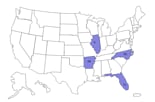
States reporting cases *
Map showing states with reported cases.
Click here to view larger map.
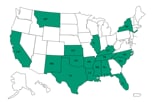
States that received recalled MPA
States that have received recalled methylprednisolone acetate product from Main Street Family Pharmacy since December 1, 2012.
Click here to view larger map.
Summary of Investigation
June 27, 2013 (No further web updates are expected)
CDC and FDA have identifiedexternal icon bacteria and fungi in unopened vials of preservative-free methylprednisolone acetate (MPA) from Main Street Family Pharmacy (MSFP) in Newbern, TN. On May 28, 2013, Main Street announced a voluntary nationwide recall of all lots of all sterile products compounded by the pharmacyexternal icon. Facilities should ensure that recalled products are no longer in use.
All 96 facilities in 17 states that received recalled MPA distributed since December 1, 2012 from Main Street Family Pharmacy (MSFP), Newbern, TN have been contacted by state or local health departments. Only 81 facilities in 15 states administered MPA, with and/or without preservative, to patients; those facilities have been provided instructions on patient notification and are responsible for notifying their patients. To date, CDC has received information that the majority of exposed patients have been notified in 62 of these 81 facilities. Notification efforts have not been initiated or neared completion in the remaining 19 facilities (1 in AR, 8 in TX, and 10 in LA).
Patients who are concerned that they might have received an injection with MPA from MSFP should contact their provider to confirm the origin of the MPA product. Patients are advised to seek medical attention if they develop symptoms suggestive of an infection resulting from an injection. Because incubation periods for fungal infections can be lengthy, patients should remain vigilant for signs and symptoms of infection for several months following a known injection with MPA from MSFP.
CDC has not received reports of infections related to any MSFP product other than preservative-free MPA. As of June 27, 26 cases meeting the CDC case definition* have been reported. The majority of cases were reported within the first week of the investigation; since June 3, after notification efforts, only 2 additional cases have been reported. The majority of cases had local skin and soft tissue inflammation at the site of injection, without an identified pathogen. There have been no reports of life-threatening infections.
Of 4 patients with positive cultures or histopathologic findings, 2 patients had Enterobacter cloacae and Klebsiella pneumoniae, 1 had Klebsiella pneumoniae, and 1 had Aspergillus sp. In addition to the risk from bacteria and fungi that have been isolated from unopened vials of MPA from MSFPexternal icon, some of these suspected infections may be due to improper handling and/or incorrect injection practices.
Clinicians are reminded that they should 1) use individual containers of compounded or preservative-free medicine for a single patient only, and 2) promptly report to MedWatchexternal icon any infection that might be related to a medication or medical device, even absent a recognized outbreak, as these reports can allow for early detection of a possible contamination event.
*Case Definition: A person who developed a suspected infection associated with injection of a product, labeled as sterile, that was distributed by the Main Street Family Pharmacy (Newbern, TN) since December 1, 2012.
June 20, 2013
There is no new update at this time.
Note: The next CDC web update is scheduled for Thursday, June 27.
June 13, 2013
CDC and FDA have identified the bacteria and fungi cultured from unopened vials of preservative-free MPA from Main Street Family Pharmacy (MSFP) in Newbern, TN. These findingsexternal icon are important reminders that healthcare providers should ensure that all recalled products from MSFP are no longer in use. In addition, complaints from patients exposed to products from MSFP should be taken seriously and should be promptly reported to both FDA MedWatchexternal icon and the health department.
In addition to the findings above, 4 of the 26 cases meeting the CDC case definition* have had bacteria or fungi detected from wounds: 2 patients had Enterobacter cloacae and Klebsiella pneumoniae, 1 had mixed bacterial culture not otherwise identified, and 1 had fungus highly suggestive of an Aspergillus sp., although further studies are needed for confirmation. Although bacteria and fungi have been isolated from unopened vials of MPA from MSFP, it is not possible to determine which infections are due to this contamination event versus other factors including improper handling and/or administration of medications at the injection facility.
Clinicians are reminded that they should 1) use individual containers of compounded or preservative-free medicine for a single-patient only, and 2) promptly report to MedWatch any infection that might be related to a medication or medical device, even absent a recognized outbreak, as these reports can allow for early detection of a possible contamination event.
June 10, 2013
As of June 10th, CDC is reporting 25 cases from four states – Arkansas, Florida, Illinois, and North Carolina. See further details below.
June 6, 2013
Suspected infections have occurred among persons who received either 80mg/mL or 40mg/mL of preservative-free methylprednisolone acetate (MPA) produced by the Main Street Family Pharmacy in Newbern, Tennessee. As of June 6th, CDC is reporting 24 cases from four states – Arkansas, Florida, Illinois, North Carolina. The majority of these persons developed skin and soft tissue infections of unclear etiology following intramuscular injection of this product. All products labeled as sterile have been voluntarily recalled by the pharmacy.
State and local health departments are working with facilities to verify the accuracy of the pharmacy’s distribution records and to determine which patients may require active outreach. Healthcare providers are contacting patients to determine if they have had possible infections stemming from injections of recalled MPA. Suspected infections that meet the criteria below* are reflected in the case count presented on this webpage. Healthcare providers are reminded that any adverse reaction following injection with a recalled product from Main Street Family Pharmacy should be reported to FDA’s Medwatchexternal icon system, which is designed to routinely collect these types of reports.
On June 6, FDA reportedexternal icon detection of microbial contamination, including bacterial and fungal, in unopened vials of preservative-free MPA. Samples are being sent to CDC for further characterization. These findings emphasize the importance for providers to continue to ensure that all recalled products are no longer in use and returned promptly. Clinicians are also reminded that individual containers of compounded or preservative free medicine are intended for single-patient use only; they should not be used as a common source of supply for multiple patients.
CDC is working closely with FDA and the health departments in affected states to monitor and to evaluate this situation. We will provide more information as it becomes available.
*Case Definition: A person who developed a suspected infection associated with injection of a product, labeled as sterile, that was distributed by the Main Street Family Pharmacy (Newbern, TN) since December 1, 2012.
June 3, 2013
CDC is aware of reports of suspected infections among persons who received either 80mg/mL or 40mg/mL of preservative-free methylprednisolone acetate (MPA) produced by the Main Street Family Pharmacy in Newbern, Tennessee. As of June 3rd, CDC is aware of 24 reported cases from four states – Arkansasexternal icon, Florida external icon, Illinoisexternal icon, North Carolinaexternal icon. The majority of these persons developed skin and soft tissue infections of unclear etiology following intramuscular injection of this product. Additional clinical information is being gathered. To date, no reports of meningitis or other life-threatening infections have been reported. All products labeled as sterile have been voluntarily recalledexternal icon by the pharmacy. CDC is not aware of infections among persons who received products other than preservative-free MPA in the above formulation from this pharmacy. State and local health departments are working with CDC and FDA to evaluate this situation.
*Case Definition: A person who developed a suspected infection associated with injection of a product, labeled as sterile, that was distributed by the Main Street Family Pharmacy (Newbern, TN) since December 1, 2012.