Volume 14, Number 6—June 2008
Dispatch
Anaplasma phagocytophilum Infection in Ixodes ricinus, Bavaria, Germany
Abstract
Anaplasma phagocytophilum DNA was detected by real-time PCR, which targeted the msp2 gene, in 2.9% of questing Ixodes ricinus ticks (adults and nymphs; n = 2,862), collected systematically from selected locations in Bavaria, Germany, in 2006. Prevalence was significantly higher in urban public parks in Munich than in natural forests.
Anaplasma phagocytophilum, an obligate intracellular bacterium, causes a febrile disease in ruminants and granulocytic anaplasmosis in dogs, horses, and humans (1). A reorganization of the order Anaplasmataceae reclassified Ehrlichia equi, E. phagocytophila, and the human granulocytic ehrlichiosis (HGE) agent to the single species A. phagocytophilum (2), which in Europe is transmitted by the sheep tick, Ixodes ricinus (3). The agent is found among the I. ricinus population in Germany; average prevalence rates are 1% to 4.5% (4,5). The English Garden, a large (3.7-km2) public park in Munich (state of Bavaria, Germany), has been suggested in 2 previous studies as a focal point for A. phagocytophilum (5,6). We investigated A. phagocytophilum in questing ticks in urban areas of Munich and focused on seasonal and geographic effects on the prevalence.
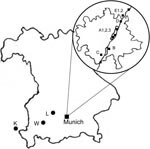
The sampling consisted of 2 phases. First, to gain an overview on the occurrence of I. ricinus, we collected questing ticks by the flagging method at 8 locations (labeled A1, A2, A3, B, C, D, E1, E2) close to the Isar River in the Munich area from May through September 2006 (Figure 1). Sites A1 and A2 were located in the city center part of the English Garden, which is enclosed by roads and houses. The vegetation of this heavily frequented area consists of groomed lawns, bushes, and deciduous trees. Site A3 was located in the northern part of the Garden, where vegetation was maintained by gardening, but bushes and trees were denser and grassland less frequently cut. The site was also used for horseback riding. Site B was a landscaped public green in the southern part of the city with groomed lawns and deciduous trees. Sites C, D, E1, and E2 were periurban riparian and deciduous forests. Three natural mixed forest sites (K, L, W) outside of Munich were sampled once (Figure 1). Ticks were registered and frozen individually at –26°C; adults were identified to species level by standard taxonomic keys (7). In the second phase, DNA was extracted from randomly chosen ticks (as available, 30 females, males, and nymphs, respectively, per month per site) with the High Pure PCR Template Preparation Kit (Roche, Mannheim, Germany) according to manufacturer’s instructions with modifications. In individual 1.5-mL tubes, each tick was crushed mechanically with a metal spatula; sterile water (200 μL) was added, and the sample was kept overnight in a 55°C water bath for complete tissue lysis. At the beginning and end of each extraction line, a negative control was added. Quality and quantity of extracted DNA were tested with a spectrophotometer (NanoDrop ND-1000, PeqLab, Erlangen, Germany). A real-time PCR targeting the msp2 gene of A. phagocytophilum (8) was performed with modifications in a Bio-Rad iCycler iQ (Bio-Rad, Munich, Germany). In a reaction volume of 25 μL, the HotStarTaq Buffer Set was used with 1.25 U HotStarTaq Polymerase (both QIAGEN, Hilden, Germany), 6 mmol/L MgCl2, 200 μmol/L each dNTP, 900 nmol/L each primer (ApMSP2f /ApMSP2r [8]), 125 nmol/L probe ApMSP2p-HEX (8), and 5.0 μL template DNA. Cycling conditions were as follows: initial activation (95°C, 15 min), 50 cycles denaturation (94°C, 15 s), and annealing–extension (60°C, 60 s). The original protocol was also used for part of the samples (8). Thirty-one DNA extracts, positive in real-time PCR, were amplified in a Thermocycler GeneAmp PCR System 2700 (Applied Biosystems, Weiterstadt, Germany) with a nested PCR (9) targeting the 16S rRNA gene, amplification of which is necessary to differentiate closely related strains (8). Negative and known positive controls were always included. After the final products were analyzed by 1.5% agarose gel electrophoresis and purified with the QIAquick PCR Purification Kit (QIAGEN) according to manufacturer’s instruction, the 497-bp fragments, without flanking primers, were sent for sequencing to MWG, Martinsried, Germany. The results were evaluated with ChromasLite (www.technelysium.com.au/chromas_lite.html), sequence homology searches made by BLASTn analysis of GenBank sequences (www.ncbi.nlm.nih.gov.library.vu.edu.au/BLAST), and multiple alignments (www.ebi.ac.uk/clustalw/index.html). The effects of month, location, stage, and sex of ticks on probability of infection were investigated with logistic regressions by using R version 2.5.0 (10); p<0.05 was regarded as significant. Due to low prevalence of A. phagocytophilum, odds ratios were interpretable as relative risks (RR). We calculated monthly prevalence with a weighted analysis, taking into account the sampling design: phase 1, a random sample, is stratified by sex, and in phase 2, a fixed number was drawn monthly at random within each sex stratum. Estimates were based on the Horvitz-Thompson estimator and corresponding 95% confidence intervals (CIs) computed by parametric bootstrap conditioning on phase 1 sample sizes (11).
A total of 9,507 ticks (4,932 adults, 3,573 nymphs, and 1,001 larvae) were collected, and adults were identified as I. ricinus. Real-time PCR was performed for 2,862 ticks (Table; online Appendix Table, available from www.cdc.gov/EID/content/14/6/972-appT.htm). With the modified protocol, atypical amplification occurred in ≈10% of samples, whereas with the original protocol, which had been tested on I. scapularis ticks, no amplification occurred. This difference suggests unspecific reactions in the modified protocol. A. phagocytophilum was detected in 5.67% of females, in 4.00% of males, and in 1.14% of nymphs (Table). The overall prevalence was 2.9% (95% CI 2.3%–3.5%). Significantly more females and males were infected than nymphs (RR = 4.906 for females, RR = 3.439 for males; p<0.001). Prevalence was significantly higher in the city parks (A1, A2, A3, B) than in natural forest areas (C, D, E1, E2, K, L, W; RR = 0.368, p<0.001). Prevalence was significantly lower in the riparian forest, Isarauen (E1, E2) in the north of Munich, than in the English Garden (A1, A2, A3) (RR = 0.314, p<0.001). Variations among the collection months, ranging from 0% to 20% for females and males and from 0 to 9.1% for nymphs (online Appendix Table, available from www.cdc.gov/EID/content/14/6/972-appT.htm), were not significant (p = 0.40).
Alignment of the partial 16S rRNA gene sequences showed that 30 sequences were 100% identical (GenBank accession no. EU490522); 1 sequence differed in 2 nucleotide positions (accession no. EU490523). The 30 homologous sequences were 100% identical to Ehrlichia sp. Frankonia 2 when compared with GenBank sequences (Figure 2) of Ehrlichia sp. Frankonia 2, A. phagocytophilum isolate X7, A. phagocytophilum isolate P80, and the prototype sequence of the HGE agent (GenBank accession nos. AF136712, AY281805, AY281794, and U02521, respectively). For Frankonia 2 and A. phagocytophilum isolate X7, the remaining sequence differed in 1 nt position. All differed in 1 nt position from the prototype HGE agent and A. phagocytophilum isolate P80 and in 2 more nt positions from P80.
Our results indicate that city parks of Munich may be focal points for A. phagocytophilum. Focal distribution depends mainly on mammalian reservoir hosts because of lack of transovarial transmission in ticks (12). Wood mice, yellow-necked mice, voles, roe, and red deer have been suggested as reservoirs in Europe (13,14). In the parks, a different reservoir host might be present. Large numbers of people and their domestic dogs pass through the parks, and the possibility of dogs acting as reservoirs for A. phagocytophilum should be investigated in further studies.
Ehrlichia sp. Frankonia 2 was first detected in adult ticks collected from domestic dogs in central Germany (15) and was later found in questing adults in Munich (5). However, neither Ehrlichia sp. Frankonia 2 nor the closely related A. phagocytophilum isolate X7 has been detected in humans or animals; thus, they can be regarded as strains of unknown pathogenicity. Future studies should aim at characterization of this strain and its possible role as a human or veterinary pathogen, as well as the identification of potential reservoir hosts in the city parks.
Mrs Silaghi is a veterinarian. She is pursuing a doctoral degree at the Institute for Comparative Tropical Medicine and Parasitology at the Ludwig-Maximilians-University, Munich, focused on A. phagocytophilum and Rickettsia spp. in Bavaria. Her main research interests are ticks and tick-borne diseases and their human and veterinary health importance.
Acknowledgment
We thank Cecilia Hizo-Teufel and Sarah Leonhard for instruction on working with tick DNA, Ingrid Pradel for excellent technical advice, Sarah Pfalzer and Sophie Neuvecelle for help with DNA extraction, Helmut Küchenhoff for valuable discussions on the statistical analysis of the data, and Lygia Friche Passos for critically reviewing the manuscript.
References
- Rikihisa Y. The tribe Ehrlichia and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308.PubMedGoogle Scholar
- Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001;51:2145–65.PubMedGoogle Scholar
- Parola P, Davoust B, Raoult D. Tick- and flea-borne rickettsial emerging zoonoses. Vet Res. 2005;36:469–92. DOIPubMedGoogle Scholar
- Hartelt K, Oehme R, Frank H, Brockmann SO, Hassler D, Kimmig P. Pathogens and symbionts in ticks: prevalence of Anaplasma phagocytophilum (Ehrlichia sp.), Wolbachia sp., Rickettsia sp., and Babesia sp. in Southern Germany. Int J Med Microbiol. 2004;293(Suppl. 37):86–92.PubMedGoogle Scholar
- Leonhard S. Untersuchungen zur Häufigkeit von Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum und Babesia spp. in Ixodes ricinus aus Bayern und Baden-Württemberg [dissertation]. München (Germany): Ludwig-Maximilians-University; 2005.
- Fingerle V, Munderloh UG, Liegl G, Wilske B. Coexistence of ehrlichiae of the phagocytophila group with Borrelia burgdorferi in Ixodes ricinus from Southern Germany. Med Microbiol Immunol (Berl). 1999;188:145–9. DOIGoogle Scholar
- Hillyard PD. Ticks of north-west Europe. Shrewsbury (UK): Field Studies Council; 1996.
- Courtney JW, Kostelnik LM, Zeidner NS, Massung RF. Multiplex real-time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol. 2004;42:3164–8. DOIPubMedGoogle Scholar
- Massung RF, Slater K, Owens JH, Nicholson WL, Mather TN, Solberg VB, Nested PCR assay for the detection of granulocytic ehrlichiae. J Clin Microbiol. 1998;36:1090–5.PubMedGoogle Scholar
- R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2007.
- Särndal C-E, Swensson B, Wretman J. Model assisted survey sampling. New York: Springer-Verlag; 1992.
- Ogden NH, Bown K, Horrocks BK, Woldehiwet Z, Bennett M. Granulocytic Ehrlichia infection in ixodid ticks and mammals in woodlands and uplands of the UK. Med Vet Entomol. 1998;12:423–9. DOIPubMedGoogle Scholar
- Liz JS, Anderes L, Sumner JW, Massung RF, Gern L, Rutti B, PCR detection of granulocytic ehrlichiae in Ixodes ricinus ticks and wild small mammals in western Switzerland. J Clin Microbiol. 2000;38:1002–7.PubMedGoogle Scholar
- Petrovec M, Bidovec A, Sumner JW, Nicholson WL, Childs JE, Avsic-Zupanc T. Infection with Anaplasma phagocytophila in cervids from Slovenia: evidence of two genotypic lineages. Wien Klin Wochenschr. 2002;114:641–7.PubMedGoogle Scholar
- Baumgarten BU, Röllinghoff M, Bogdan C. Prevalence of Borrelia burgdorferi and granulocytic and monocytic ehrlichiae in Ixodes ricinus ticks from southern Germany. J Clin Microbiol. 1999;37:3448–51.PubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 14, Number 6—June 2008
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Cornelia Silaghi, Institute for Comparative Tropical Medicine and Parasitology, Ludwig-Maximilians-University, Leopoldstr. 5, 80802 Munich, Germany;
Top