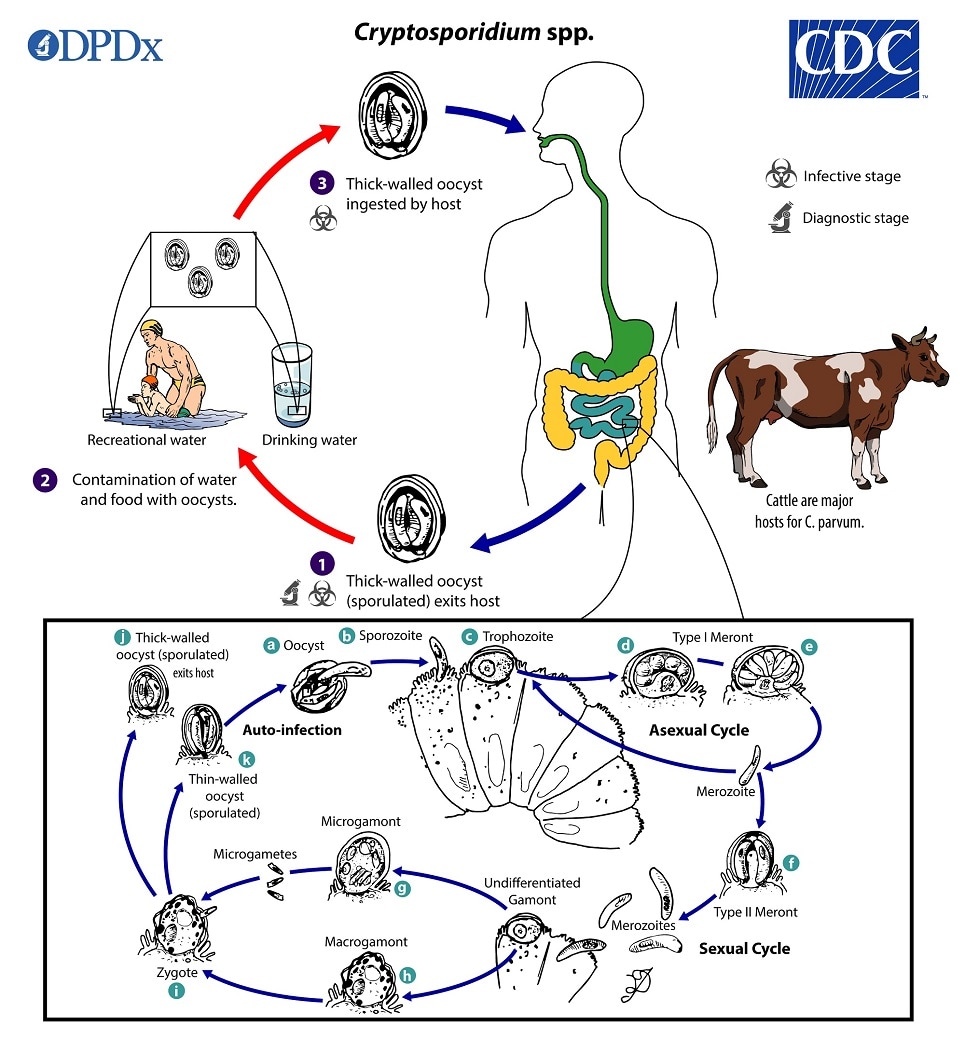
Cryptosporidiosis
[Cryptosporidium spp.]
Causal Agents
Many species and genotypes of the apicomplexan protozoan Cryptosporidium can infect humans and have a wide range of host animals. Zoonotic species and genotypes of Cryptosporidium are those transmitted from animal hosts to humans, and non-zoonotic species and genotypes are host-adapted without evidence of transmission from animals to humans. Cryptosporidium parvum (formerly known as C. parvum genotype II) and C. hominis (formerly known as C. parvum genotype I) are the leading causes of human cryptosporidiosis. C. meleagridis, C. felis, C. canis, C. ubiquitum, C. cuniculus, C. viatorum, Chipmunk genotype I, Cryptosporidium mink genotype, and C. muris can also infect humans.
Life Cycle:
Sporulated oocysts, containing 4 sporozoites, are excreted by the infected host through feces  (and possibly other routes such as respiratory secretions). Transmission of Cryptosporidium spp. occurs mainly through ingestion of fecally contaminated water (e.g., drinking or recreational water) or food (e.g., raw milk) or following direct contact with infected animals or people
(and possibly other routes such as respiratory secretions). Transmission of Cryptosporidium spp. occurs mainly through ingestion of fecally contaminated water (e.g., drinking or recreational water) or food (e.g., raw milk) or following direct contact with infected animals or people  . Following ingestion (and possibly inhalation) by a suitable host
. Following ingestion (and possibly inhalation) by a suitable host  , excystation
, excystation  occurs. The sporozoites are released and parasitize the epithelial cells (
occurs. The sporozoites are released and parasitize the epithelial cells (  ,
,  ) of the gastrointestinal tract (and possibly the respiratory tract). In these cells, usually within the brush border, the parasites undergo asexual multiplication (schizogony or merogony) (
) of the gastrointestinal tract (and possibly the respiratory tract). In these cells, usually within the brush border, the parasites undergo asexual multiplication (schizogony or merogony) (  ,
,  ,
,  ) and then sexual multiplication (gametogony) producing microgamonts (male)
) and then sexual multiplication (gametogony) producing microgamonts (male)  and macrogamonts (female)
and macrogamonts (female)  . Upon fertilization of the macrogamonts by the microgametes (
. Upon fertilization of the macrogamonts by the microgametes (  ) that rupture from the microgamont, oocysts develop and sporulate in the infected host. Zygotes give rise to two different types of oocysts (thick-walled and thin-walled). Thick-walled oocysts are excreted from the host into the environment
) that rupture from the microgamont, oocysts develop and sporulate in the infected host. Zygotes give rise to two different types of oocysts (thick-walled and thin-walled). Thick-walled oocysts are excreted from the host into the environment  , whereas thin-walled oocysts are involved in the internal autoinfective cycle and are not recovered from stools
, whereas thin-walled oocysts are involved in the internal autoinfective cycle and are not recovered from stools  . Oocysts are infectious upon excretion, thus enabling direct and immediate fecal-oral transmission. Extracellular stages have been reported, but their relevance in the overall life cycle is unclear.
. Oocysts are infectious upon excretion, thus enabling direct and immediate fecal-oral transmission. Extracellular stages have been reported, but their relevance in the overall life cycle is unclear.
Hosts:
Cryptosporidium can infect a wide range of vertebrate hosts, including birds, reptiles, and mammals. Many species and genotypes are host-adapted, but human cases caused by species and genotypes that are pathogens in other mammals or animals have been reported (e.g. C. meleagridis). predominantly infects humans and is generally considered anthroponotic, though sporadic reports in animal hosts exist. Zoonotic subtype families of C. parvum implicated in human infections are commonly associated with cattle, particularly calves.
Geographic Range:
Zoonotic and non-zoonotic Cryptosporidium spp. and genotypes are ubiquitous worldwide. Outbreaks of cryptosporidiosis have been and continue to be reported in several countries . Cryptosporidiosis outbreaks in the U.S. have been linked to swimming pools, water playgrounds, and other swimming venues; unpasteurized cider, juice, and milk; contact with animals; childcare settings; camps; and ill food handlers.
Clinical Presentation
Infection with Cryptosporidium spp. and genotypes results in a wide range of signs and symptoms. The incubation period is an average of 7 days (range: 2–10 days). Immunocompetent patients may present with diarrheal illness that is self-limiting, typically resolving within 2–3 weeks. Immunocompromised patients may have more severe complications, such as life-threatening malabsorption and wasting. Diarrheal illness may be accompanied by fever or fatigue). While the small intestine is primarily affected, extraintestinal cryptosporidiosis (e.g., in the pulmonary or biliary tract, rarely in the pancreas) has been reported.
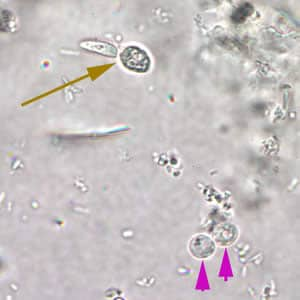
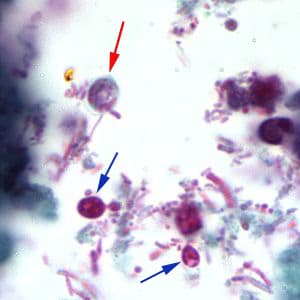
Cryptosporidium sp. oocysts in a wet mount.
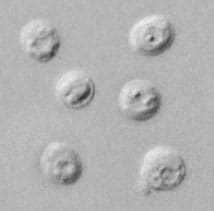
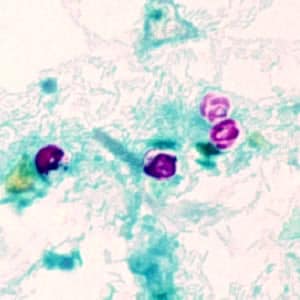
Cryptosporidium sp. oocysts stained with trichrome.
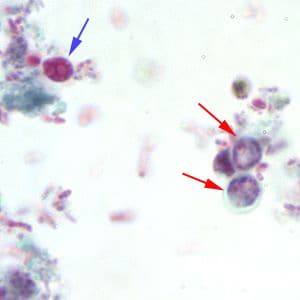
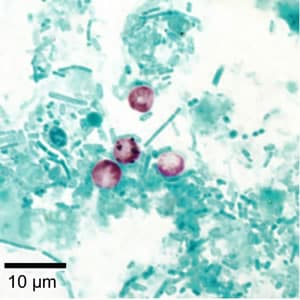
Cryptosporidium sp. oocysts stained with modified acid-fast.
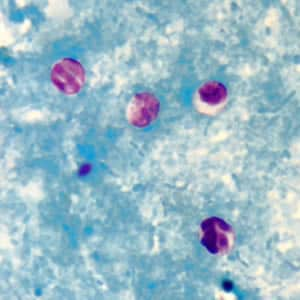
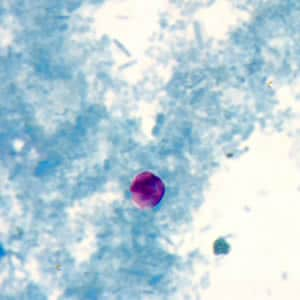
Cryptosporidium sp. oocysts unstained on a slide stained with modified acid-fast.
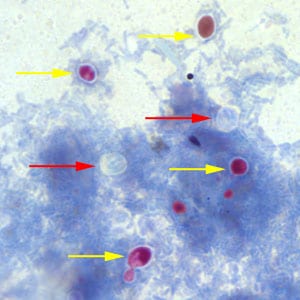
Cryptosporidium sp. oocysts stained with Ziehl-Neelsen modified acid-fast.
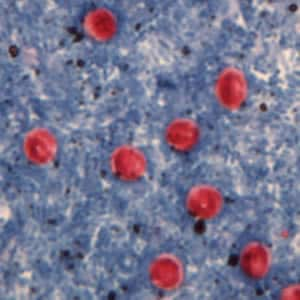
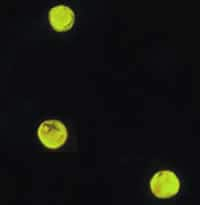
Cryptosporidium parvum oocysts stained with the fluorescent stain auramine-rhodamine.
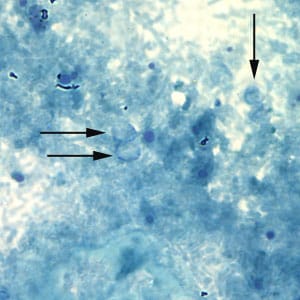
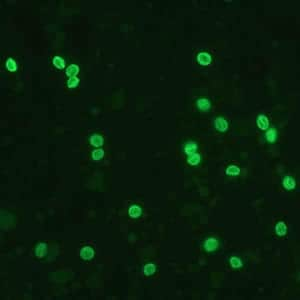
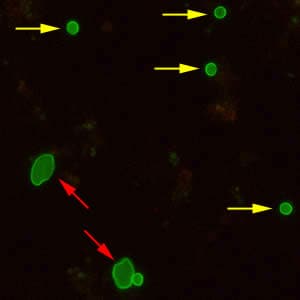
Oocysts of C. parvum and cysts of Giardia duodenalis labeled with immunofluorescent antibodies.
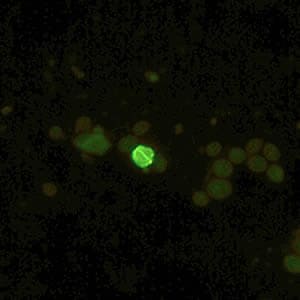
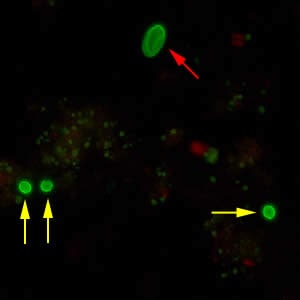
Laboratory Diagnosis
Acid-fast staining methods, with or without stool concentration, are most frequently used in clinical laboratories. Immunofluorescence microscopy has the greatest sensitivity and specificity, followed closely by enzyme immunoassays (EIA). Molecular methods (e.g., polymerase chain reaction [PCR)]) are increasingly used in reference diagnostic laboratories, since they can identify Cryptosporidium at the species level.
Specimen processing
Stool specimens may be preserved in 10% buffered formalin (see “Laboratory Safety”), or suspended in a storage medium composed of aqueous potassium dichromate (2.5% w/v, final concentration). Formalin-based fixatives are not recommended if molecular testing will be performed*. Because the number of oocysts can vary, even in liquid stools samples, multiple stool specimens should be tested before reporting a negative diagnostic interpretation. To maximize recovery of oocysts, stool specimens should be concentrated prior to microscopic examination. Formalin-ethyl acetate sedimentation is the recommended stool concentration method. Given their small size and mass, cryptosporidial oocysts can become trapped in the ether or ethyl acetate plug and fail to sediment properly . Increased centrifugation speed or time (500 x g, 10 minutes) might be warranted when attempting to recover cryptosporidial oocysts. Resolution of cryptosporidial infections is accompanied by increasing numbers of non-acid-fast, oocyst “ghosts.” Such oocysts might not float or sediment as expected, leading to false-negative results.
* Formalin is routinely used in clinical settings as a fixative of various specimen types. However, because of formalin’s unfavorable effects on nucleic acids, certain fixatives/preservatives are not recommended for molecular detection, including formalin, sodium acetate-acetic acid-formalin (SAF), and low-viscosity polyvinyl alcohol (LV-PVA).
Immunoassays
Direct fluorescent antibody [DFA], EIA, and rapid immunochromatographic assays are commercially available in the U.S. for the diagnosis of cryptosporidiosis. Several kits are combined tests for Cryptosporidium, Giardia, and Entamoeba histolytica. Immunodetection of antigens on the surface of organisms in stool specimens using DFA is highly sensitive and specific.
Commercial EIA tests for the detection of Cryptosporidium antigens in fresh or frozen stool specimens and also in stool specimens preserved in formalin or fixed in sodium acetate-acetic acid-formalin (SAF) are available in the microplate format. Concentrated or polyvinyl alcohol-treated (PVA) specimens are unsuitable for testing with available antigen detection EIA kits.
Laboratories that use EIA kits and rapid format assays need to be aware of potential problems with false positives and interpret results with caution.
Molecular Methods
Multipathogen Molecular Panels
Several commercial multipathogen gastrointestinal illness panels are available that include Cryptosporidium.. The advantage of these technologies is they deliver the often high sensitivity and specificity of molecular assays (e.g. PCR), without the need for highly trained molecular biologists. Also, infections can be detected even in the absence of clinical suspicion.
Molecular Typing
Molecular methods, such as those developed and used by CryptoNet, are the only methods able to differentiate Cryptosporidium species and genotypes. These are used in epidemiological investigations and genetic typing and not for clinical diagnosis. Multiple individual protocols comprise the standardized method for Cryptosporidium genotyping and subtyping .
Laboratory Safety
Standard precautions for the processing of stool specimens apply.
Cryptosporidium spp. oocysts are hardy and immediately infectious, so they present an infection risk for laboratory workers via accidental ingestion (or possibly aerosolization); therefore extra precautions must be taken. Primary containment (e.g., biosafety cabinet) and/or personal protective equipment (PPE; e.g., face shield) should be used when working with specimens that might contain viable Cryptosporidium spp. All paper towel litter and other disposable materials should be autoclaved or similarly disinfected before disposal. Reusable laboratory items can be washed and disinfected in a laboratory dishwasher by using a detergent containing chlorine and the “sanitize” cycle. Alternatively, contaminated items may be immersed for approximately one hour in a water bath preheated to 50°C and washed thereafter in a detergent/disinfectant solution.
All spills and potential surface contamination with oocysts should be disinfected using the following protocol: After removing organic material from the contaminated surface (e.g., by using a conventional laboratory detergent/cleaner) and absorbing the bulk of the spill with disposable paper towels, flood and completely cover the surface with undiluted 3% hydrogen peroxide. Dispense hydrogen peroxide repeatedly, as needed, to keep affected surfaces covered and wet/moist for approximately 30 minutes. Absorb residual hydrogen peroxide with disposable paper towels, and allow surfaces to dry thoroughly (10–30 minutes) before use.
Suggested Reading
Checkley, W., White, A.C., Jaganath, D., Arrowood, M.J., Chalmers, R.M., Chen, X.M., Fayer, R., Griffiths, J.K., Guerrant, R.L., Hedstrom, L. and Huston, C.D., 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. The Lancet Infectious Diseases, 15 (1), pp.85-94.
Ryan, U., Fayer, R. and Xiao, L., 2014. Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology, 141 (13), pp.1667-1685.
DPDx is an educational resource designed for health professionals and laboratory scientists. For an overview including prevention, control, and treatment visit www.cdc.gov/parasites/.