Diagnosis and Detection
It is almost impossible to distinguish a single patient with cholera from a patient infected by another pathogen that causes acute watery diarrhea without testing a stool sample. A review of clinical features of multiple patients who are part of a suspected outbreak of acute watery diarrhea can be helpful in identifying cholera because of the rapid spread of the disease.
While management of patients with acute watery diarrhea is similar regardless of the illness, it is important to identify cholera because of the potential for a widespread outbreak.
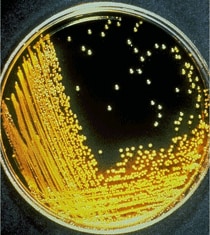
Vibrio cholerae growing on thiosulphate citrate bile salt sucrose (TCBS) agar plates
How to Diagnose
Isolation and identification of Vibrio cholerae serogroup O1 or O139 by culture of a stool specimen remains the gold standard for the laboratory diagnosis of cholera.
Cary Blair media is ideal for transport, and the selective thiosulfate–citrate–bile salts agar (TCBS) is ideal for isolation and identification. Reagents for serogrouping Vibrio cholerae isolates are available in all state health department laboratories in the U.S. Commercially available rapid test kits are useful in epidemic settings but do not yield an isolate for antimicrobial susceptibility testing and subtyping, and should not be used for routine diagnosis.
In many countries where cholera is not uncommon, but access to diagnostic laboratory testing is difficult, WHO recommends the following clinical definition be used for suspected cholera cases.
Suspected cholera case
- In areas where a cholera outbreak has not been declared: Any patient 2 years old or older presenting with acute watery diarrhea and severe dehydration or dying from acute watery diarrhea.
- In areas where a cholera outbreak is declared: any person presenting with or dying from acute watery diarrhea.
WHO also recommends the following definition for confirmed cholera cases.
Confirmed cholera case
A suspected case with Vibrio cholerae O1 or O139 confirmed by culture or PCR and, in countries where cholera is not present or has been eliminated, the Vibrio cholerae O1 or O139 strain is demonstrated to be toxigenic.
Rapid Tests
In areas with limited or no laboratory testing, the Crystal® VC dipstick rapid test can provide an early warning to public health officials that an outbreak of cholera is occurring. However, the sensitivity and specificity of this test is not optimal. Therefore, it is recommended that fecal specimens that test positive for V. cholerae O1 and/or O139 by the Crystal® VC dipstick always be confirmed using traditional culture-based methods suitable for the isolation and identification of V. cholerae.
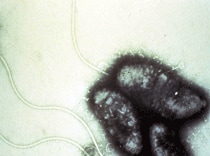
Electron micrograph of Vibrio cholerae
Detection in the U.S.
Cholera is a nationally reportable disease in the U.S. All isolates should be sent to CDC via state health department laboratories for cholera toxin-testing and subtyping.