Chapter 13: Rotavirus
Authors: Daniel C. Payne, PhD, MSPH; Umesh D. Parashar, MBBS, MPH
Disease Description
Before the advent of rotavirus vaccines, rotavirus was the most common cause of severe gastroenteritis in infants and young children. Rotavirus-vaccinated populations have experienced dramatic decreases in rotavirus infections and transmission, so norovirus is now the most common pediatric viral gastroenteritis.[1] The clinical spectrum of rotavirus illness ranges from mild, watery diarrhea of limited duration to severe diarrhea with vomiting and fever that can result in dehydration with shock, electrolyte imbalance, and on very rare occasions for U.S. children, death. Following an incubation period of 1–3 days, the illness often begins abruptly, and vomiting often precedes the onset of diarrhea. Gastrointestinal symptoms generally resolve in 3–7 days. Up to one-third of patients have a temperature of >102°F (>39°C). Severe, dehydrating rotavirus infection occurs primarily among unvaccinated children 3–35 months of age. [2,7]
Rotaviruses are shed in high concentrations in the stools of infected children and are transmitted primarily by the fecal-oral route, both through close person-to-person contact and through fomites.[8] Rotaviruses also are probably transmitted by other modes, such as respiratory droplets and fecally contaminated food and water.[9] Rotavirus is highly communicable, with a small infectious dose of < 100 virus particles;[10] the World Health Organization therefore reports that hygienic measures[11] and improved sanitation[12,13] are not likely to lead to significant declines in rotavirus burden in unvaccinated populations. For this reason, rotavirus vaccines have been identified as the optimal strategy to decrease the burden associated with severe and fatal rotavirus diarrhea worldwide.[14]
Repeated exposures occur from birth to old age, but natural and/or vaccine-induced immunity renders the majority of infections mild or asymptomatic following natural infection or vaccination.[15] Children who are immunocompromised sometimes experience severe, prolonged, and even fatal rotavirus gastroenteritis.[16-19] Rotavirus infections do occur among U.S. adults but tend to be mild or even asymptomatic infections. In some adults, especially those who have underlying medical conditions or who are older adults, rotavirus can still cause severe gastroenteritis requiring medical care.[20, 21]
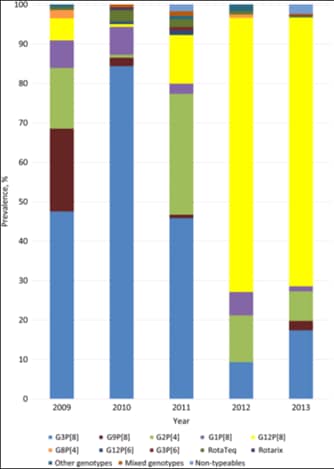
Figure 1. Rotavirus genotype prevalence among children <5 years old: New Vaccine Surveillance Network (NVSN), 2009–2013[26]
Background
Rotaviruses are nonenveloped RNA viruses belonging to the Reoviridae family. The viral nucleocapsid is composed of 3 concentric shells that enclose 11 segments of double-stranded RNA. The outermost layer contains 2 structural viral proteins (VPs): VP4, the protease-cleaved protein (P protein) and VP7, the glycoprotein (G protein). These 2 proteins define the serotype of the virus and are considered critical to vaccine development because they are targets for neutralizing antibodies that might be important for protection. Because the 2 gene segments that encode these proteins can segregate independently, a typing system consisting of both P and G types has been developed. Several animal species (e.g., primates, cows, horses, pigs, sheep) are susceptible to rotavirus infection and suffer from rotavirus diarrhea, but common animal rotavirus serotypes differ from prevalent human strains. Although human rotavirus strains that possess a high degree of genetic homology with animal strains have been identified, animal-to-human transmission of whole virions appears to be uncommon. Most human rotaviruses having some genetic similarity to animal rotaviruses appear formed by reassortment of one or more animal rotavirus genes into a human rotavirus during a mixed infection in vivo.
In rare instances, [22] reassortment between RotaTeq vaccine component strains of genotypes P7[5]G1 and P1A[8]G6 have been observed to occur during human in vivo replication. This vaccine-derived reassortant has been demonstrated to be transmissible, and is capable of causing symptomatic gastroenteritis.[22,23] Typically in the post-licensure vaccine era, this reassortant has been detected among 1-3% of detected rotavirus surveillance cases that have undergone sequencing.[24]
In the United States, viruses containing 6 distinct P and G combinations are most prevalent: P[8]G1, P[4] G2, P[8] G3, P[8] G4, P[8] G9, and P[6] G9, although more than 40 rare or regional strains have been identified in the United States and globally.[25] In recent years P[8],G12[26] has become the predominant strain among severe rotavirus gastroenteritis cases in the United.States., although secular variation by geographic region may still exist (Figure 1).[27,29]
Vaccination
Rotavirus vaccine descriptions and clinical trial results:
RotaTeq:
In 2006, RotaTeq, a live, oral, human-bovine reassortant rotavirus vaccine produced by Merck and Company (Whitehouse Station, New Jersey) was recommended by the Advisory Committee on Immunization Practices (ACIP) for routine vaccination of U.S. infants. Three doses of this vaccine are recommended to be administered at 2, 4, and 6 months of age, concurrently with other vaccines given at this age.[2] RotaTeq contains 5 reassortant rotaviruses developed from human and bovine parent rotavirus strains that express human outer capsid proteins of 5 common circulating strains (G1, G2, G3, G4, and P[8] [subgroup P1A]).
RotaTeq has been tested in 2 phase III trials,[30,31] including a large-scale clinical trial of more than 70,000 infants enrolled primarily in the United States and Finland. The efficacy of 3 doses of RotaTeq against G1-G4 rotavirus gastroenteritis of any severity was 74% (95% confidence interval [CI] = 67%, 80%) and against severe G1-G4 rotavirus gastroenteritis was 98% (CI = 88%, 100%). RotaTeq was observed to be effective against each targeted serotype and reduced the incidence of medical office visits by 86% (CI = 74%, 93%), emergency department (ED) visits by 94% (CI = 89%, 97%), and rotavirus gastroenteritis hospitalizations by 96% (CI = 91%, 98%). Efficacy against all gastroenteritis hospitalizations of any etiology was 59% (CI = 52%, 65%) for the period beginning after dose.[2]
Rotarix:
In 2008, ACIP recommended that Rotarix (produced by GlaxoSmithKline Biologicals, Rixensart, Belgium), be used for routine vaccination of U.S. infants. This live vaccine contains the attenuated monovalent G1, P[8] human rotavirus strain and is recommended by the manufacturer to be orally administered in 2 doses to infants at 2 and 4 months of age.
In a large clinical trial of more than 63,000 infants from 11 Latin American countries, Rotarix was found to be safe and highly immunogenic. During the first year after vaccination, the efficacy (as defined by the Vesikari 20-point scoring system) of 2 doses of Rotarix against hospitalization due to severe rotavirus was 85% and 100% against more severe rotavirus gastroenteritis.[32] After 2 years of follow-up the vaccine demonstrated 83% (CI=73%, 90%) efficacy in preventing rotavirus-related hospitalizations. Rotarix was protective against hospitalizations due to all causes of gastroenteritis (42% protection for the first year, CI=27%, 54%). Rotarix provided protection against a broad range of rotavirus serotypes during the 2-year study period, including against the less common G9, P[8] strain.
In a randomized, double-blind, placebo-controlled study conducted in 6 European countries, Rotarix was observed to be highly immunogenic. The efficacy of Rotarix through 1 rotavirus season against any grade of severity of rotavirus gastroenteritis was 87% (CI = 80%, 92%) and against severe rotavirus gastroenteritis (as defined by a score ≥11 on the Vesikari scale) was 96% (CI=90%, 99%). Rotarix reduced hospitalizations for all-cause gastroenteritis regardless of presumed etiology by 75% (CI = 46%, 89%). The efficacy of Rotarix through 2 rotavirus seasons against severe rotavirus gastroenteritis was 90% (CI= 85%, 94%), and in reducing hospitalizations was 96% (CI= 84%, 100%).[33,34]
No clinical trial has yet compared the efficacy of Rotarix against that of RotaTeq, and ACIP offers no vaccine preference. For harmonization of vaccination administration scheduling, the ACIP now recommends that, for both vaccines, the maximum age for dose 1 is 14 weeks and 6 days (previous recommendation: 12 weeks), and the maximum age for the last dose of rotavirus vaccine is 8 months and 0 days (previous recommendation: 32 weeks).[2]
Vaccine coverage
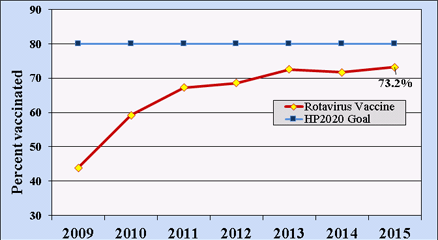
Rotavirus vaccination rates (for either vaccine) among U.S. children 19–35 months of age have been estimated using systematic sampling methods by the National Immunization Survey since 2009. By 2015, nearly three-quarters of U.S. children within this age range had received a full course of rotavirus vaccines, below the Healthy People 2020 goal of 80% coverage (Figure 2).[35]
Post-licensure vaccine impact
During the pre-rotavirus vaccine era, it was estimated that 410,000 physician visits; 205-272,000 ED visits; and 55,000–70,000 hospitalizations were attributable to rotavirus infections in U.S. children, costing approximately $1 billion annually (Figure 2).[36] The licensure and approval by ACIP of rotavirus vaccines (RotaTeq in 2006 and Rotarix in 2008) brought dramatic overall declines in rotavirus disease burden among U.S. children, which have been consistently observed in various surveillance efforts.
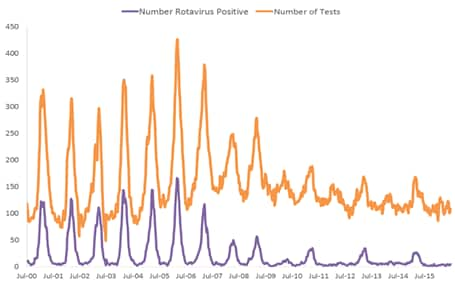
Numerous post-licensure publications documenting rotavirus vaccine impact have demonstrated great declines in the incidence of rotavirus gastroenteritis. Since 2000, the National Respiratory and Enteric Virus Surveillance System (NREVSS), a network of 17 laboratories, has continuously provided laboratory reports of rotavirus tests performed and positive results, showing that the number of laboratory-detected rotavirus-attributable infections has plummeted since rotavirus vaccine introduction (Figure 3).[37] In an 18-state analysis of hospital discharge data (accounting for 49% of the U.S. population) from 2000 to 2008, acute, all-cause gastroenteritis hospitalization rates for children <5 years of age were calculated. Compared with the median rate for the 2000–2006 rotavirus seasons, the rates for 2007 and 2008 were 16% and 45% lower, respectively. These analyses were among the first to find that RotaTeq vaccine introduction was associated with a dramatic reduction in rotavirus gastroenteritis among U.S. children almost immediately following rotavirus vaccine introduction.[38]
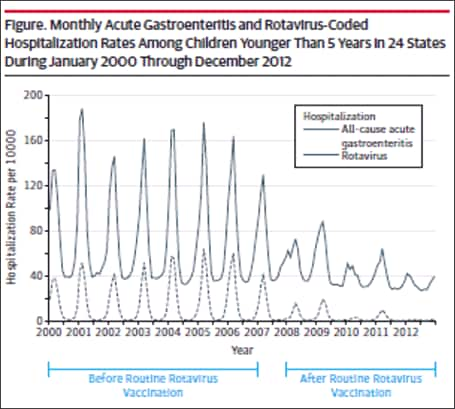
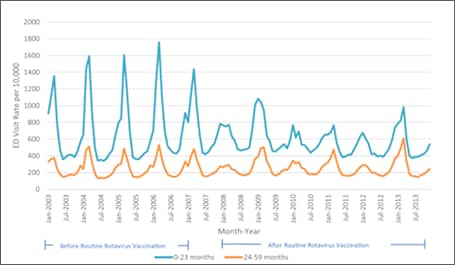
Subsequent analyses in other databases and clinical settings have reinforced those findings. Using rotavirus gastroenteritis hospital discharge codes, Leshem et al[39] assessed the State Inpatient Databases, an administrative dataset representing 74% of U.S. hospitalizations for children <5 years of age in 24 states. Compared with the pre-vaccine era (2000–2006), a 63–94% decline in rotavirus-coded hospitalizations was observed in the 2008-2012 post-vaccine era (Figure 4).[39] Post-vaccine reductions were also observed in rotavirus gastroenteritis emergency department (ED) visits. An assessment of rotavirus gastroenteritis ED visits was made of the State Emergency Department Visit database, representing 13,350 U.S. emergency department visits for children <5 years of age in 10 states.[40] Again, large declines (albeit slightly less dramatic) were observed in rotavirus-coded emergency department visits in the 2003-2006 pre-vaccine era (Figure 5). Active surveillance from the NVSN observed 73%–99% post-licensure reductions from the pre-vaccine licensure baseline period in laboratory-confirmed rotavirus hospitalizations from 2011–2015 among children under 3 years of age.[41]
Each of these analyses also revealed an interesting biennial disease trend, regardless of clinical setting. This biennial trend emerged immediately following rotavirus vaccine introduction, whereby rotavirus incidence was sharply curtailed during the winters of even-numbered years (e.g., 2008, 2010, 2012) and rose again during odd-numbered years (2009, 2011, 2013, etc.), albeit to a level remaining below that observed during the pre-vaccine era. Predicted in rotavirus modeling analyses,[42] this observed biennial trend in clinical and laboratory reports is believed to be due to annual fluctuations in susceptible children and the efficiency of rotavirus transmission. Rotavirus vaccination rates of eligible U.S. children appear to be high enough to temporarily limit disease transmission, but since fewer than 80% have received vaccine, those unvaccinated children who remain susceptible to disease accumulate over time until a period when rotavirus transmission in a population can again occur with efficiency.[29]
Rotavirus vaccines have been observed to provide indirect protective benefits, even among those who have not been vaccinated. Analyses of large hospital discharge databases show a statistically significantly lower rate of rotavirus- or unspecified-gastroenteritis hospitalization among household members having a vaccinated child.[43]
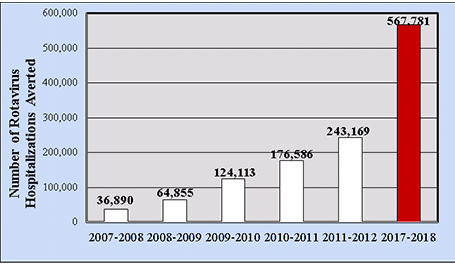
In terms of direct medical costs, during the pre-vaccine era the median medical cost per hospitalized child was $3,581, from analyses of actively collected billing records of laboratory-confirmed U.S. cases.[44] The rotavirus hospitalization rate in 2006 was 22.1/10,000, with an estimated annual national cost of $91 million. Increased costs were associated with study site, age <3 months of age, underlying medical conditions, and an atypical acute gastroenteritis presentation. For ED visits, the pre-vaccine median medical cost per child was $574, and the ED visit rate was 291/10,000, resulting in an estimated annual national cost of $192 million (2009 dollars). In a cohort analysis of commercially-insured hospital claims data from the Market Scan administrative database, by 2017–2018 the cumulative number of rotavirus-attributable hospitalizations averted by rotavirus vaccination among children <5 years of age in the United States should top 567,000 hospitalizations (Figure 6).[45]
Post-licensure vaccine effectiveness
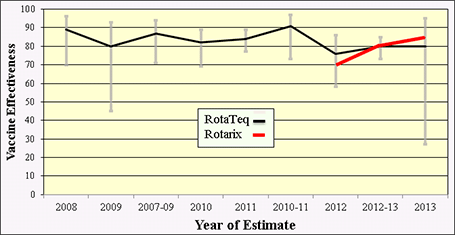
Similar to the consistent results demonstrating rotavirus vaccine impact in the United States, several field studies have been conducted to determine vaccine effectiveness (VE) in the United States. At least 7 studies assessing vaccine performance have involved active, prospective surveillance in clinical settings with laboratory confirmation of rotavirus cases. These studies of both RotaTeq and Rotarix vaccines, collectively, reveal a consistent vaccine effectiveness of approximately 70–90% (Figure 7).
A case-control study conducted in Texas by Boom et al.[35] of 3-dose RotaTeq vaccine effectiveness in children age-eligible to receive vaccination during the 2007–08 and 2008–09 rotavirus seasons showed a combined VE of 84% (CI = 70%,92%). In a follow-up case-control study at this institution of children 15 days-23 months of age enrolled during February–June 2008, even partial immunization with RotaTeq provided protection against rotavirus disease, with an effectiveness of 69% (CI = 13%,89%) for a single dose, 81% (CI = 13%,96%) for two doses, and 88% (CI = 68%, 96%) for a full course of 3 doses.[46]
RotaTeq vaccine effectiveness was evaluated in a multi-site study during the first 3 years following licensure by Staat et al. and the NVSN,[47] which found that 3-dose effectiveness against G1-G4 rotavirus hospitalizations and ED was 94.5% (CI = 91%,97%),. Vaccine effectiveness estimates were comparable between the first and second years of life, and similar across observed rotavirus strains.
Desai et al.[48] conducted a case-control study of children 8 weeks to 3 years of age in Connecticut during January 2006–August 2009. The adjusted VE for a complete 3-dose course of RotaTeq® was 96% (CI=29%,100%) when calculated using hospitalized controls and 99% (CI=78%,100%) using community controls.[48]
As the incidence of severe rotavirus declined around the country, assessing rotavirus vaccine effectiveness increasingly required multi-site, active surveillance capture of cases. Studies by Payne et al.,[49,51] Cortese et al.,[43] and Immergluck et al.[50] utilized large surveillance systems to produce robust, stratified estimates of the performance of both licensed rotavirus vaccines.
In a geographically-diverse NVSN study involving 7 U.S. medical institutions conducted over 2 years (2012–13), Payne et al.[49] reported the aggregate VE for ≥1 dose of any rotavirus vaccine as 80% (95% CI = 74%,85%) against rotavirus hospitalizations and ED visits. In this aggregate analysis, a complete 3-dose course of RotaTeq had a VE of 80% (CI = 74%,84%), and a complete 2-dose course of RV1 had a VE of 80% (CI = 68%,88%). Results stratified by inpatient and ED clinical settings were similar to these aggregate estimates. Investigators did not find any statistical difference in performance by vaccine type, for fully vaccinated children or for children receiving any vaccine dose. Statistically significant VE was observed through the seventh year of life for RotaTeq and through the third year of life for Rotarix, which was approved by ACIP approximately 2 years after RotaTeq. Effectiveness estimates against rotavirus infections categorized as mild, moderate, and severe were 67% (CI = 48%,79%), 78% (CI = 70%,85%), and 84% (CI = 71%,92%), respectively. Furthermore, both vaccines appeared to protect against the 4 predominant circulating rotavirus strains, including rotavirus strain G12P[9] which has recently emerged as the predominant U.S. strain[26], without statistical difference.
Immergluck et al. studied children enrolled in the hospital or ED at a single, large surveillance site in Georgia in 2013.[50] A complete course of RotaTeq and Rotarix vaccinations were found to be 80% (CI = 27%,95%) and 84% (CI = 38%, 96%) protective, respectively, against rotavirus hospital or ED visits for children 8-23 months of age. For children older than 24 months of age, RotaTeq and Rotarix were 87% (CI = 22%,98%) and 82% (CI = 41%,95%) protective against these clinical visits, respectively.
The ACIP recommends that a child’s rotavirus vaccine series be completed with the same product whenever possible, but allows interchanging vaccine types if the product used for a previous dose(s) is not available or is unknown. In such situations, ACIP recommends that “If any dose in the series was RV5 or the vaccine product is unknown for any dose in the series, a total of 3 doses of rotavirus vaccine should be administered.” In a study comparing these 2 vaccines concurrently administered in childhood populations, the complete 3-course rotavirus vaccination regimen with mixed RotaTeq and Rotarix vaccines retained a statistically significant level of protection against severe rotavirus gastroenteritis that was nearly identical to that observed for complete, single vaccine type regimens.[51]
Other unexpected benefits of rotavirus vaccination
Indirect protective benefits
Indirect protective benefits of rotavirus vaccination to unvaccinated individuals were not studied during the clinical trials of either currently licensed rotavirus vaccine. However, U.S. rotavirus surveillance empirically indicated that reductions in rotavirus disease burden among older, unvaccinated children likely result from indirect protection conferred by younger, vaccinated children within the household and community. These indirect benefits may even extend to older children and adults, groups not previously thought to have substantial rotavirus burden.[29,52,53]
Preventive association of rotavirus vaccination with seizures
Rotavirus infection is not limited to the intestine; it is associated with antigenemia and viremia, and the potential for systemic illness exists.[7] Wild-type rotavirus has been detected in cerebrospinal fluid (CSF) in cases of seizure occurring with diarrhea[54-57] and neurologic illnesses have been attributed to rotavirus infection in numerous studies and case reports.[57–63]
Studies in the United States,[64] Spain,[65] and Australia[66] demonstrated that children vaccinated with rotavirus vaccine have a reduction in the risk of seizures requiring hospitalization or ED care compared with unvaccinated children during the year following vaccination. In the United States, this represented an approximate 20% reduction in seizure risk (both febrile and afebrile) associated with rotavirus vaccination.
Several mechanisms may explain this protective association between rotavirus vaccination and seizures, although there is little clinical information to inform our understanding of this pathway. The most likely mechanism is that vaccination directly prevents systemic rotavirus infection, including extra-intestinal complications involving the central nervous system.[67] Vaccination may also prevent secondary effects of rotavirus infection, including calcium channel fluctuations resulting in neurotransmitter dysregulation[68–70] and/or a rotavirus-related elevation of nitric oxide in CSF that induces neurotoxicity.[71,72]
- Vulnerable populations: In 2009 and 2010, ACIP specifically contraindicated RotaTeq and Rotarix vaccines, respectively, in infants diagnosed with severe combined immunodeficiency. Consultation with an immunologist or infectious disease specialist is advised for infants with known or suspected altered immunocompetence before rotavirus vaccine is administered.[73] The ACIP recommendation does not include routine rotavirus vaccination of those having a history of intussusception or history of a severe allergic reaction after a previous rotavirus vaccine dose, to a vaccine component, or to latex (if administering human attenuated rotavirus vaccine).[2]
Furthermore, ACIP specifies that the vaccine should be administered to clinically stable preterm infants who fulfill the chronological age requirements (i.e., 6 weeks through 14 weeks and 6 days of age for dose 1) at or after discharge from the neonatal intensive care unit (or nursery). However, infants born prematurely or with underlying conditions are at increased risk of severe rotavirus disease and associated complications and may not be age-eligible for vaccination at the time of their discharge.[74] Recent data finding no evidence of nosocomial rotavirus vaccine-strain transmission in a neonatal intensive care unit (IR = 0.0 [95% CI = 0.0–1.3]) cases per 1,000 patient-days at risk) suggest that delaying rotavirus vaccination until the time of hospital discharge (due to a theoretical risk of nosocomial transmission of live rotavirus vaccine virus) may be unnecessary in institutions with acceptable infection control standards.[75]
Innate host factors influencing immunity to rotavirus
Histo-blood group antigens (HBGAs) are carbohydrates expressed on the mucosal epithelia of hman respiratory, genitourinary, and digestive tracts that serve as host receptor sites necessary for bacterial/viral attachment and cellular entry and, therefore, infection. The expression of secretor antigen production (including FUT2) on HBGA’s can be inactivated by single nucleotide polymorphisms (SNPs), and those individuals having genetically inactivated FUT2 production are termed non-secretors.
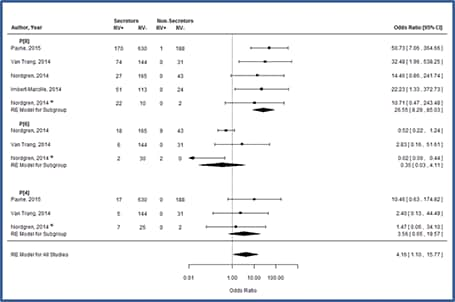
Figure 8. Host genetic susceptibility to enteric viruses: A systematic review and meta-analysis[81]
Relationships between HBGA functionality and enteric infections are accepted for norovirus[76], and recently, correlations between rotavirus infections and secretor status have been observed in Vietnamese,[77] French,[78] Burkinabe,[79] Nicaraguan,[79] and U.S.[80] children hospitalized with acute gastroenteritis, each demonstrating strong, statistically significant correlations between these HGBA polymorphisms and the absence of rotavirus gastroenteritis.[79] Subsequent meta-analyses indicate that this association (Odds Ratio=25) is driven by increased likelihood of infection with P[8] rotaviruses among secretors.[81] Differences in susceptibility to P[8] rotaviruses may also suggest that non-secretors respond less well to vaccination, a topic undergoing further assessment (Figure 8).
Mathematical modeling of rotavirus trends
Several mathematical modeling analyses have been conducted to better understand the effects of rotavirus vaccination upon disease trends. Using a deterministic, age-structured model in a developed country setting, Atchinson, et al.[82] computed that short-term age-specific fluctuations in rotavirus incidence and age distributions were consequences of a rotavirus vaccination program and were unrelated to waning immunity or falling vaccine coverage. Pitzer et al.[42] calculated that the mean age of severe rotavirus cases would increase with higher vaccine coverage due to delays in primary rotavirus infection, that the spatiotemporal characteristics of rotavirus epidemics are largely related to accumulations of fully susceptible individuals by geographic location, and that the reduction in rotavirus prevalence would be greater than that predicted by the direct effect of vaccination alone. These modeling predictions have matched published empirical observations.[36]
Vaccine safety
A modestly elevated risk of intussusception (~1–2 cases per 100,000 vaccine recipients) among rotavirus vaccine recipients has been noted in some international settings and in the United States. In the U.S. State Inpatient Database, an increase in the intussusception hospitalization rate was observed in children 8 to 11 weeks old, when the majority of first doses of vaccine are given. This finding is consistent with other U.S. studies. The Vaccine Safety Datalink (VSD) analysis found the attributable risk of intussusception after the administration of 2 doses of Rotarix to be 5.3 per 100,000 infants vaccinated.[83] VSD also found an upper limit for the attributable risk of 1 intussusception case per 65,287 RotaTeq dose-1 recipients.[84] Given the magnitude of declines in rotavirus disease compared with this small increase in intussusception, the benefits of rotavirus vaccination are considered to outweigh the increased risk of intussusception.
Importance of Surveillance
With rotavirus vaccines routinely used by the U.S. childhood immunization program, it remains important to conduct surveillance to:
- monitor the impact of vaccination in reducing morbidity and mortality from rotavirus disease over time;
- evaluate vaccine effectiveness in field use and identify and determine the causes of possible vaccine failure;
- monitor the possible emergence of rotavirus strains that might escape vaccination;
- identify population groups that might not be adequately covered by vaccination; and
- continue to monitor the safety of rotavirus vaccines.
Surveillance efforts should focus on monitoring trends of severe rotavirus disease, such as rotavirus hospitalizations or emergency room visits, at the national level and through more intensive efforts at sentinel sites. In addition to severe and medically-attended disease surveillance, viral strain surveillance is also important to evaluate whether strain variability is a secular phenomenon or whether it is the result of a potential selection of rotavirus serotypes through vaccine pressures.
Current active, passive, and laboratory-based national rotavirus surveillance includes the following:
- NVSN: Active rotavirus surveillance activities through NVSN began in the 2005–06 rotavirus season with 3 original sites and have continued prospectively with 7 sites. Acute gastroenteritis cases are identified and additional epidemiological and clinical information is collected from parental interviews and medical chart reviews. Stool specimens are tested for rotavirus antigen at each study site, and CDC laboratories type all positive specimens. Analyses are conducted to estimate disease burden and to assess rotavirus vaccine effectiveness in field use.
- NREVSS and the National Rotavirus Strain Surveillance System: Laboratory-based sentinel surveillance activities through the NREVSS monitor temporal and geographic patterns associated with the detection of several viruses, including rotavirus. Approximately 17 laboratories located in state and local health departments, universities, and hospitals have participated in the NREVSS since 2000. Participating laboratories report the total number of fecal specimens submitted for rotavirus testing and the number that tested positive for rotavirus on a weekly basis to CDC. A subset of 10–12 NREVSS laboratories participate in NRSSS. These NRSSS laboratories submit a representative sample of rotavirus-positive fecal specimens to CDC for strain characterization by molecular methods.
- Secondary analysis of national health utilization datasets: National estimates of the burden of rotavirus disease have been derived primarily through review of passive surveillance data on diarrhea mortality, hospitalizations, and ambulatory visits collected by the National Center for Health Statistics (e.g., the National Hospital Discharge Survey, the National Ambulatory Care Survey). In this approach, a set of International Classification of Diseases, Ninth and Tenth Volumes, Clinical Module (ICD–9 and 10–CM) codes have been first used to identify events attributable to acute gastroenteritis. Then, the unique epidemiologic characteristics of rotavirus gastroenteritis (i.e., predilection for children 4–35 months of age, marked winter seasonality) have been used to estimate the proportion of diarrhea events attributable to rotavirus. A rotavirus-specific ICD code was introduced in 1992. One validation study found that this code had a high positive predictive value (i.e., coded events were highly likely to be true cases) but had a sensitivity of <50%. Nonetheless, by applying a variety of ICD codes related to acute gastroenteritis to these large databases and accounting for the seasonal and age-specific distributions of rotavirus incidence, it is possible to deduce large-scale rotavirus disease patterns and impacts.
Disease Reduction Goals
Healthy People 2020 states an 80% goal for rotavirus vaccination coverage.[85]
Case Definitions
Definitive diagnosis of rotavirus gastroenteritis requires laboratory confirmation of infection. Currently, no case definition for rotavirus gastroenteritis is approved by the Council of State and Territorial Epidemiologists (CSTE; www.cste.org). Active surveillance being conducted at sentinel sites by CDC defines a confirmed case of rotavirus gastroenteritis as a child with diarrhea (≥3 loose stools in 24 hours) OR vomiting (≥1 episodes in 24 hours) and with detection of rotavirus in a fecal specimen by a standard assay (e.g., commercially available enzyme immunoassays).
Laboratory Testing
It is not possible to diagnose rotavirus infection by clinical presentation because the clinical features of rotavirus gastroenteritis do not differ from those of gastroenteritis caused by other pathogens. Confirmation of rotavirus infection by laboratory testing is necessary for reliable rotavirus surveillance and can be useful in clinical settings to avoid inappropriate use of antimicrobial therapy.
Rotavirus is shed in high concentration in the stool of children with gastroenteritis and a fecal specimen is the preferred specimen for diagnosis. The most widely available method for detection of rotavirus antigen in stool is an enzyme immunoassay (EIA) directed at an antigen common to all group A rotaviruses. Several commercial EIA kits are available that are inexpensive, easy to use, rapid, and highly sensitive (approximately 90–100%), making them suitable for rotavirus surveillance and clinical diagnosis.[50];’ Polyacrylamide gel electrophoresis and silver staining is about as sensitive as EIA but is very labor intensive.[51] Latex agglutination is less sensitive and specific than EIA but is still used in some settings.[50] Other techniques, including electron microscopy, reverse transcription polymerase chain reaction (RT-PCR), nucleic acid hybridization, sequence analysis, and culture are used primarily in research settings.
Specimen collection
Specimen collection and shipping are important steps in obtaining laboratory diagnosis or disease confirmation. Guidelines have been published for specimen collection and handling for viral and microbiologic agents. Information is also available on using CDC laboratories as support for reference and disease surveillance; this includes
- a central website for requesting lab testing;
- the form required for submitting specimens to CDC (See Appendix 23, Form # CDC 0.5034);
- information on general requirements for shipment of etiologic agents (Appendix 24 [4 pages]) —although written to guide specimen submission to CDC, this information may be applicable to submission of specimens to other laboratories); and
- the CDC Infectious Diseases Laboratories Test Directory, which contains not only a list of orderable tests for that institution, but also detailed information on appropriate specimen types, collection methods, specimen volume, and points of contact.
Rotavirus serotypes can be determined directly from rotavirus-positive stool specimens using both EIA and RT-PCR methods. Monoclonal antibody-based EIA techniques have been invaluable in defining 4 globally common serotypes (G1-G4) that represent >90% of the circulating strains and make up 4/5 serotypes in the RotaTeq vaccine.[86,87] More recently, molecular methods—including multiplexed, semi-nested RT-PCR genotyping and nucleotide sequencing—have been developed as a surrogate for serotypes and have become widely used to identify the most common and several uncommon rotavirus G and P genotypes.88-90] Nucleotide sequencing has been extensively used to identify uncommon strains and genetic variants that cannot be identified by RT-PCR genotyping and to confirm the results of genotyping methods.
Reporting and Case Notification
Rotavirus gastroenteritis is not a nationally reportable disease and notification is not required by CDC. Contact the state health department for reporting requirements in your state.
Case Investigation
Case investigations are usually not warranted, except perhaps during outbreaks or in the case of deaths or other serious manifestations of rotavirus infections. Because diarrheal outbreaks can be caused by many pathogens, a laboratory investigation for the causative agent that includes viral, bacterial, and parasitic agents should be considered for gastroenteritis cases seeking medical attention.
Control
Routine immunization of infants is anticipated to be the most effective public health intervention for population-wide rotavirus infection control. Post-exposure vaccine prophylaxis is not a recommended strategy in response to an outbreak of rotavirus gastroenteritis.
References
- Payne DC, Vinje J, Szilagyi PG, et al. Norovirus and medically attended gastroenteritis in U.S. children. New Engl J Med 2013;368:1121–30. doi: 10.1056/NEJMsa1206589
- CDC. Prevention of rotavirus gastroenteritis among infants and children: recommendation of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58(RR-2):1–25.
- Gurwith M, Wenman W, Hinde D, Feltham S, Greenberg H. A prospective study of rotavirus infection in infants and young children. J Infect Dis 1981;144(3):218–24. doi: 10.1093/infdis/144.3.218
- Kapikian AZ, Chanock RM. Rotaviruses. In: Straus SE, editor. Fields virology: Volume 2. 3rd ed. Philadelphia: Lippincott-Raven; 1996. p.1657–708.
- Rodriguez WJ, Kim HW, Brandt CD, et al. Longitudinal study of rotavirus infection and gastroenteritis in families served by a pediatric medical practice: clinical and epidemiologic observations. Pediatr Infect Dis J 1987;6(2):170–6.
- Glass RI, Kilgore PE, Holman RC, et al. The epidemiology of rotavirus diarrhea in the United States: surveillance and estimates of disease burden. J Infect Dis 1996;174(Suppl 1):S5–11. doi: 10.1093/infdis/174.Supplement_1.S5
- Carlson JAK, Middleton PJ, Szymanski MT, Huber J, Petric M. Fatal rotavirus gastroenteritis: an analysis of 21 cases. Am J Dis Child 1978;132(5):477–9.
- Butz AM, Fosarelli P, Kick J, Yolken R. Prevalence of rotavirus on high-risk fomites in daycare facilities. Pediatrics 1993;92(2):202–5.
- Dennehy PH, Nelson SM, Crowley BA, Saracen CL. Detection of rotavirus RNA in hospital air samples by polymerase chain reaction (PCR). Pediatr Res 1998;43:143. doi: 10.1203/00006450-199804001-00849
- American Academy of Pediatrics. Rotavirus infections. In: Pickering LK, editor. Red Book: 2003 report of the Committee on Infectious Diseases. 26th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2003. p.534–6.
- Mrukowicz J, Szajewska H, Vesikari T. Options for the prevention of rotavirus disease other than vaccination. J Pediatr Gastroenterol Nut 2008;46:S32–7. doi: 10.1097/MPG.0b013e31816f79b0
- Jin S, Kilgore PE, Holman RC, Clarke MJ, Gangarosa EJ, Glass RI. Trends in hospitalizations for diarrhea in United States children from 1979–1992: estimates of the morbidity associated with rotavirus. Pediatr Infect Dis J 1996;15(5):397–404.
- Parashar UD, Holman RC, Clarke MJ, Bresee JS, Glass RI. Hospitalizations associated with rotavirus diarrhea in the United States, 1993 through 1995: surveillance based on the new ICD-9-CM rotavirus-specific diagnostic code. J Infect Dis 1997;177(1):13–7. doi: 10.1086/513808
- World Health Organization. Rotavirus vaccines WHO position paper: January 2013 – Recommendations. Vaccine 2013;31:6170–1. doi: 10.1016/j.vaccine.2013.05.037
- Pickering LK, Bartlett AV III, Reves RR, Morrow A. Asymptomatic excretion of rotavirus before and after rotavirus diarrhea in children in day care centers. J Pediatr 1988;112(2):361–5.
- Saulsbury FT, Winkelstein JA, Yolken RH. Chronic rotavirus infection in immunodeficiency. J Pediatr 1980;97(1):61–5.
- Yolken RH, Bishop CA, Townsend TR. Infectious gastroenteritis in bone-marrow transplant recipients. New Engl J Med 1982;306(17):1009–12. doi: 10.1056/NEJM198204293061701
- Troussard X, Bauduer F, Gallet E, et al. Virus recovery from stools of patients undergoing bone marrow transplantation. Bone Marrow Transplant 1993;12(6):573–6.
- Liakopoulou E, Mutton K, Carrington D, et al. Rotavirus as a significant cause of prolonged diarrhoeal illness and morbidity following allogeneic bone marrow transplantation. Bone Marrow Transplant 2005;36:691–4.
- Bresee JS, Marcus R, Venezia RA, et al. The etiology of severe acute gastroenteritis among adults visiting emergency departments in the United States. J Infect Dis 2012;205:1374–81. doi: 10.1093/infdis/jis206
- Hardy D. Epidemiology of rotaviral infection in adults. Rev Infect Dis 1987;9(3):461–9. doi: 10.1093/clinids/9.3.461
- Payne DC, Edwards KE, Bowen MD, et al. Sibling transmission of vaccine-derived rotavirus (RotaTeq) associated with rotavirus gastroenteritis. Pediatrics 2010;125(2):e438–41. doi: 10.1542/peds.2009-1901
- Hemming M, Vesikari T. Detection of Rotateq vaccine-derived, double-reassortant rotavirus in a 7-year-old child with acute gastroenteritis. Pediatr Infect Dis J 2014; 33:655-6. doi: 10.1097/INF.0000000000000221
- Boom JA, Sahni L, Payne DC, et al. Symptomatic infection and detection of vaccine and vaccine-reassortant rotavirus strains in 5 children: A case series. J Infect Dis 2012;206(8):1275–9. doi: 10.1093/infdis/jis490
- Gentsch JR, Laird AR, Bielfelt B, et al. Serotype diversity and reassortment between human and animal rotavirus strains: implications for rotavirus vaccine programs. J Infect Dis 2005;192(Suppl 1):S146–59. doi: 10.1086/431499
- Bowen MD, Mijatovic-Rustempasic S, Esona MD, et al. Rotavirus strain trends during the post-licensure vaccine era: United States, 2008–2013. J Infect Dis 2016;214(5):732–8. doi: 10.1093/infdis/jiw233
- Hull JJ, Teel EN, Kerin TK, et al. United States rotavirus strain surveillance from 2005–2008: genotype prevalence before and after vaccine introduction. Pediatr Infect Dis J 2011;30(1):S42–7. doi: 10.1097/INF.0b013e3181fefd78
- Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics 2010; 125(2):e199–207. doi: 10.1542/peds.2009-1021
- Payne DC, Staat MA, Edwards KM, et al. Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 U.S. counties, 2006–2009. Clin Infect Dis. 2011;53(3):245–53. doi: 10.1093/cid/cir307
- Block SL, Vesikari T, Goveia MG, et al. Efficacy, immunogenicity, and safety of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine at the end of shelf life. Pediatrics 2007;119(1):11–8. doi: 10.1542/peds.2006-2058
- Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006;354(1):23–33. doi: 10.1056/NEJMoa052664
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006;354(1):11–22. doi: 10.1056/NEJMoa052434
- Vesikari T, Karvonen A, Puustinen L, et al. Efficacy of RIX 4414 live attenuated human rotavirus vaccine in Finnish infants. Pediatr Infect Dis J 2004;23(10):937–43.
- Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomized, double-blind controlled study. Lancet 2007;370(9601):1757–63. doi: 10.1016/S0140-6736(07)61744-9
- Hill HA, Elam-Evans LD, Yankey D, et al. Vaccination coverage among children aged 19-35 months— United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65(39):1065-71. doi: 10.15585/mmwr.mm6539a4
- Parashar UD, Gibson CJ, Bresee JS, et al. Rotavirus and severe childhood diarrhea. Emerg Infect Dis 2006;12(2):304–6. doi: 10.3201/eid1202.050006
- Aliabadi N, Tate JE, Haynes AK, et al.; CDC. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination—United States, 2000–2014. MMWR Morb Mortal Wkly Rep 2015;64:337–42.
- Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among U.S. children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 U.S. states. J Infect Dis 2010;201(11):1617–24. doi: 10.1086/652403
- Leshem E, Tate JE, Steiner CA, et al. Acute gastroenteritis hospitalizations among U.S. children following implementation of the rotavirus vaccine. JAMA 2015;313(22):2282–4. doi:10.1001/jama.2015.5571
- Shah MP, Tate JE, Steiner CA, et al. Decline in emergency department visits for acute gastroenteritis among children in 10 U.S. states after implementation of rotavirus vaccination, 2003 to 2013. Pediatr Infect Dis J 2016;35(7):782–6. doi: 10.1097/INF.0000000000001175
- Payne DC. A decade of documenting the impact of rotavirus vaccination in the United States: Understanding the post-rotavirus vaccine introduction era. Presented at the 5th European Expert Meeting on Rotavirus Vaccination (EEROVAC); 2017 Mar 21, Utrecht, Netherlands.
- Pitzer VE, Viboud C, Simonsen L, et al. Demographic variability, vaccination, and the spatiotemporal dynamics of rotavirus epidemics. Science 2009;325(5938):290–4. doi: 10.1126/science.1172330
- Cortese MM, Dahl RM, Curns AT, et al. Protection against gastroenteritis in U.S. households with children who received rotavirus vaccine. J Infect Dis 2015;211(4):558-62. doi: 10.1093/infdis/jiu503
- Kilgore A, Donauer S, Edwards KM, et al. Rotavirus-associated hospitalization and emergency department costs and rotavirus vaccine program impact. Vaccine 2013;31(38):4164–71. doi: 10.1016/j.vaccine.2013.06.085
- Leshem E, Moritz RE, Curns AT, et al. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007–2011). Pediatrics 2014;134(1):15–23. doi: 10.1542/peds.2013-3849
- Boom JA, Tate JE, Sahni LC, et al. Sustained protection from pentavalent rotavirus vaccination during the second year of life at a large, urban United States pediatric hospital. Pediatr Infect Dis J 2010;29(12):1133–5. doi: 10.1097/INF.0b013e3181ed18ab
- Staat MA, Payne DC, Donauer S, et al. Effectiveness of the pentavalent rotavirus vaccine against rotavirus disease. Pediatrics 2011;128(2):e267–75. doi: 10.1542/peds.2010–3722
- Desai SN, Esposito DB, Shapiro ED, Dennehy PH, Vázquez M. Effectiveness of rotavirus vaccine in preventing hospitalization due to rotavirus gastroenteritis in young children in Connecticut, USA. Vaccine 2010;28(47):7501–6. doi: 10.1016/j.vaccine.2010.09.013
- Payne DC, Selvarangan R, Azimi PH, et al. Long-term consistency in U.S. rotavirus vaccine protection: RV5 and RV1 vaccine effectiveness in U.S. children, 2012–2013. Clin Infect Dis 2015;61(12):1792–9. doi: 10.1093/cid/civ872
- Immergluck LC, Parker TC, Jain S, et al. Sustained effectiveness of monovalent and pentavalent rotavirus vaccines in children. J Pediatr 2016;172:116-20. doi: 10.1016/j.jpeds.2016.01.042
- Payne DC, Sulemana I, Parashar UD. Evaluation of effectiveness of mixed rotavirus vaccine course for severe rotavirus gastroenteritis. JAMA Pediatr, 2016;170(7):708–10. doi:10.1001/jamapediatrics.2016.0014
- Buttery JP, Lambert SB, Grimwood K, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s National Childhood vaccine schedule. Pediatr Infect Dis J 2011;30(1):S25–9. doi: 10.1097/INF.0b013e3181fefdee
- Lopman BA, Curns AT, Yen C, et al. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis 2011;204(7):980–6. doi: 10.1093/infdis/jir492
- Dickey M, Jamison L, Michaud L, et al. Rotavirus meningoencephalitis in a previously healthy child and a review of the literature. Pediatr Infect Dis J 2009;28(4):318–21. doi: 10.1097/INF.0b013e31818ddbe9
- Iturriza-Gómara M, Auchterlonie IA, Zaw W, Molyneaux P, Desselberger U, Gray J. Rotavirus gastroenteritis and central nervous system (CNS) infection: characterization of the VP7 and VP4 genes of rotavirus strains isolated from paired fecal and cerebrospinal fluid samples from a child with CNS disease. J Clin Microbiol 2002;40(12):4797–9. doi: 10.1128/JCM.40.12.4797-4799.2002
- Nishimura S, Ushijima H, Nishimura S, et al. Detection of rotavirus in cerebrospinal fluid and blood of patients with convulsions and gastroenteritis by means of the reverse transcription polymerase chain reaction. Brain Dev 1993;15(6):457–9. doi: 10.1016/0387-7604(93)90088-P
- Abe T, Kobayashi M, Araki K, et al. Infantile convulsions with mild gastroenteritis. Brain Dev 2000;22(5):301–6. doi: 10.1016/S0387-7604(00)00111-X
- Wang YC, Hung KL. Benign seizures associated with mild diarrhea: clinical analysis of 20 cases. Acta Paed Sin 1993;34(6):451–7.
- Contino MF, Lebby T, Arcinue EL. Rotaviral gastrointestinal infection causing afebrile seizures in infancy and childhood. Am J Emerg Med 1994;12(1):94–5.
- Lin SC, Hsu HY, Wang PJ, et al. Rotavirus gastroenteritis associated with afebrile seizure in childhood. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1996;37(3):204–7.
- Hung JJ, Wen HY, Yen MH, et al. Rotavirus gastroenteritis associated with afebrile convulsion in children: clinical analysis of 40 cases. Chang Gung Med J 2003;26(9):654–9.
- Narchi H. Benign afebrile cluster convulsions with gastroenteritis: an observational study. BMC Pediatr 2004;4:2. doi: 10.1186/1471-2431-4-2
- Thompson MJ, Gowdie PJ, Kirkwood CD, et al. Rotavirus cerebellitis: New aspects to an old foe? Ped Neurol 2012;46(1):48–50. doi: 10.1016/j.pediatrneurol.2011.10.002
- Payne DC, Baggs J, Zerr D, Curns AT, Weintraub E, Parashar UD. Protective association between rotavirus vaccination and childhood seizures in the year following vaccination in U.S. children. Clin Inf Dis 2013;58(2):173–7. doi: 10.1093/cid/cit671
- Pardo-Seco J, Cebey-López M, Martinón-Torres, et al. Impact of rotavirus vaccination on childhood hospitalization for seizures. Pediatr Infect Dis J 2015;34(7):769–73. doi: 10.1097/INF.0000000000000723
- Sheridan SL, Ware RS, Grimwood K, et al. Febrile seizures in the era of rotavirus vaccine. J Pediatric Infect Dis Soc2016;5(2):206–9. doi: 10.1093/jpids/piu097
- DiFazio MP, Braun L, Freedman S, et al. Rotavirus-induced seizures in childhood. J Child Neurol 2007;22(12):1367–40. doi: 10.1177/0883073807307083
- Weclewicz K, Svensson L, Kristensson K. Targeting of endoplasmic reticulum-associated proteins to axons and dendrites in rotavirus-infected neurons. Brain Res Bull 1998;46(4):353–60.
- Morris A, Scott J, Ball J, Zeng CQ, O’Neal WK, Estes MK. NSP4 elicits age-dependent diarrhea and Ca(2+) mediated I(-) influx into intestinal crypts of CF mice. Am J Physiol 1999;277(2):G431–4.
- Tian P, Ball J, Zeng C, Estes MK. The rotavirus nonstructural glycoprotein NSP4 possesses membrane destabilization activity. J Virol 1996;70(10):6973–81.
- Kawashima H, Inage Y, Ogihara M, et al. Serum and cerebrospinal nitrite/nitrate levels in patients with rotavirus induced convulsions. Life Sci 2004;74(11):1397–1405.
- Zhang J, Dawson V, Dawson T, et al. Nitric oxide activation of poly(ADP-Ribose) synthetase in neurotoxity. Science 1994;263(5147):687–9.
- CDC. Addition of severe combined immunodeficiency as a contraindication for administration of rotavirus vaccine. MMWR Morb Mortal Wkly Rep 2010;59(22):687–8.
- Dennehy PH, Cortese MM, Begue RE, et al. A case-control study to determine risk factors for hospitalization for rotavirus gastroenteritis in U.S. children. Pediatr Infect Dis J 2006;25(12):1123–31. doi: 10.1097/01.inf.0000243777.01375.5b
- Hofstetter AM, Lacombe K, Klein EJ, et al. Rotavirus vaccine uptake and lack of nosocomial spread in hospitalized infants. Pediatrics 2017; submitted.
- Hutson AM, Atmar RL, Graham DY, Estes MK. Norwalk virus infection and disease is associated with ABO histo-blood group type. J Infect Dis 2002;185(9):1335–7. doi: 10.1086/339883
- Trang NV, Vu HT, Le NT, Huang P, Jiang X, Anh DD. Association between norovirus and rotavirus infection and histo-blood group antigen types in Vietnamese children. J Clin Microbiol 2014; 52(2):1366–74. doi: 10.1128/JCM.02927-13
- Imbert-Marcille BM, Barbé L, Dupé M, et al. A FUT2 gene common polymorphism determines resistance to rotavirus A of the P[8] genotype. J Infect Dis 2014;209(8):1227–30. doi: 10.1093/infdis/jit655
- Nordgren J, Sharma S, Bucardo F, et al. Both Lewis and secretor status mediate susceptibility to rotavirus infections in a rotavirus genotype dependent manner. Clin Infect Dis 2014;59(11):1567–73. doi: 10.1093/cid/ciu633
- Payne DC, Currier R, Staat MA, et al. Epidemiologic association between FUT2 secretor status and severe rotavirus gastroenteritis in U.S. children. JAMA Pediatr 2015; 169(11):1040–5. doi: doi:10.1001/jamapediatrics.2015.2002
- Khambhampati A, Payne DC, Constantini V, Lopman BA. Host genetic susceptibility to enteric viruses: a systematic review and meta-analysis. Clin Infect Dis 2016;62(1):11–8. doi: 10.1093/cid/civ873
- Atchison C, Edmunds J, Patel M, et al. Natural dynamics of mass rotavirus vaccination. Presented at the 9th International Rotavirus Symposium: 2010 Sep 4, Johannesburg, South Africa.
- Weintraub ES, Baggs J, Duffy J, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med 2014;370(6):513–9. doi: 10.1056/NEJMoa1311738
- Shui IM, Baggs J, Patel M, et al. Risk of intussusception following administration of a pentavalent rotavirus vaccine in U.S. infants. JAMA 2012;307(6):598–604. doi:10.1001/jama.2012.97
- U.S. Department of Health and Human Services. IID-7.10. Achieve and maintain an effective coverage level of 2 or more or 3 or more doses rotavirus vaccine among children by age 19 to 35 months. In: Healthy People 2020. Washington, DC: U.S. Department of Health and Human Services; 2017.
- Taniguchi K, Urasawa T, Morita Y, Greenberg HB, Urasawa S. Direct serotyping of human rotavirus in stools using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis 1987;155(1):1159–66. doi: 10.1093/infdis/155.6.1159
- Coulson, B, Unicomb LE, Pitson GA, Bishop RF. Simple and specific enzyme immunoassay using monoclonal antibodies for serotyping human rotaviruses. J Clin Microbiol 1987;25(3):509–5.
- Gunasena S, Nakagomi O, Isegawa Y, et al. Relative frequency of VP4 gene alleles among human rotaviruses recovered over a 10 year period (1982-1991) from Japanese children with diarrhea. J Clin Microbiol 1993;31(8):2195–97.
- Gouvea V, Glass RI, Woods P, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol 1990;28(2):276–82.
- Gentsch JR, Glass RI, Woods P, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol 1992;30(6):1365–73.