

|

|

Volume
2:
No. 2, April 2005
ORIGINAL RESEARCH
Secular Trends in Age at Menarche, Smoking, and Oral
Contraceptive Use Among Israeli Girls
Gabriel Chodick, PhD, MHA, Michael Huerta, MD, MPH, Ran D. Balicer,
MD, Nadav Davidovitch, MD, MPH, Itamar Grotto, MD, MPH
Suggested citation for this article: Chodick G, Huerta
M, Balicer RD, Davidovitch N, Grotto I. Secular trends in age at
menarche, smoking, and oral contraceptive use among Israeli
girls. Prev Chronic Dis [serial online]. 2005 Apr
[date cited]. Available from: URL: http://www.cdc.gov/pcd/issues/2005/
apr/04_0063.htm.
PEER REVIEWED
Abstract
Introduction
The improved nutrition and socioeconomic status of the
population in industrialized countries has resulted in a decrease
in the mean age at menarche. This trend raises the question of
whether cigarette smoking and the use of oral contraceptives,
health behaviors often adopted during adolescence, may also be
starting at a younger age. Cigarette smoking and use of oral
contraceptives are a public health concern because they pose an
increased risk for development of chronic diseases, particularly
in combination. This study was designed to identify secular
trends in age at menarche, at first cigarette, and at first use
of oral contraceptives among a large population-based sample of
young Israeli women and to assess whether these trends are
associated with sociodemographic factors.
Methods
A systematic, population-based survey used data obtained from
female recruits to the Israel Defense Force from 1986 to 2000.
During the study period, 11,392 questionnaires were collected
from Jewish women aged 18 to 19 years. Participants were interviewed
concerning geographic origin and level of education,
father’s geographic origin and level of education, current
smoking status, use of oral contraceptives, and recalled age at
first menstruation, first cigarette, and first use of oral
contraceptives.
Results
Reported mean age (± SD) at menarche showed a monotonic
trend of decreasing over time, from 13.41 (± 1.30) years for
women born before 1970 to 13.03 (± 1.28) years for those
born after 1978 (P < .001). Women born after 1978 were
twice as likely to experience menarche by the age of 11 as those born prior to 1970 (odds ratio 2.0; 95% confidence
interval, 1.41–2.82). Significant trends toward younger age at
first use were observed for cigarettes and oral
contraceptives.
Conclusion
The trends of earlier age at menarche, first cigarette, and
first use of oral contraceptives suggest health behaviors among
young women that may herald increased chronic disease morbidity
in the future. These trends indicate the need for further
investigation and preventive measures aimed at this
population.
Back to top
Introduction
The onset of puberty in women is marked by numerous changes,
both physiological and behavioral, the most marked of which is
the commencement of menstruation (1). The mean age at menarche
reflects numerous health aspects of a population, including the
timing of sexual maturation, growth and nutritional status, and
environmental conditions (2). In the United States, the mean age
at menarche decreased from more than 14 years prior to 1900 (3)
to less than 12.5 years currently (2), although racial
differences in maturational timing have been observed
consistently in nationally representative and clinical samples,
across time and using different study designs (4,5). Recently
published data on the incidence of breast cancer among a large
cohort of French women provide further evidence that the overall
risk of breast cancer increases with younger age at menarche.
Among premenopausal women, those with a history of menarche at
age 11 or younger were at increased risk for breast cancer when
compared with those with menarcheal onset at age 15 or older (relative risk 1.52; 95% confidence interval [CI],
1.03–2.22) (6). Secular trends in the average age at menarche
may, in fact, affect the future epidemiology of breast
cancer.
Puberty is often accompanied by the adoption of new behaviors
such as alcohol drinking, cigarette smoking, and the use of oral
contraceptives (OC) for pregnancy prevention with the
commencement of sexual activity. In addition to the
well-established data on the risks to health from cigarette
smoking, substantial data are available on the excessive risk for
cardiovascular diseases among smokers who use OC (7). A recently
published report on mortality among women who used OC reveals more than a
doubled risk for death from all causes among heavy smokers (8). Thus, data on
trends in onset of puberty and behavioral aspects of adolescence are important
to public health policy makers. However, few data on age of menarche in Israel
are currently available. No population-based survey has been undertaken on this
issue; a single published cross-sectional study was conducted more than 20 years ago (9).
The objectives of this study were to describe secular trends
in age at menarche, at first cigarette, and at initial use of OC
among a large population-based sample of young Israeli women and
to assess whether changes in these parameters over time are
associated with sociodemographic factors.
Back to top
Methods
Study population
The study population was derived from an ongoing large-scale
prospective survey of health behavior and attitudes routinely
administered among a fixed proportion of Israel Defense Force
(IDF) recruits. Because military service is mandatory in Israel,
the survey provides a population-based sample of the young adult
population, excluding ultra-Orthodox Jews and Arabs, who are
largely exempted from service. The sampling process is systematic
and is based on a predetermined combination of digits of the
participant’s serial number, as previously described
(10,11). Ninety-five percent of all female study participants are
aged 18 to 19 at inclusion. The present study targeted all
females recruited from 1986, when questions on age at menarche
were first introduced, through 2000.
Data collection
Study subjects were asked to participate on the day of
recruitment. Trained nurses from the IDF Health Surveillance
Section interviewed participants. Participants were asked about
their father’s geographic origin (divided into two
categories: West [i.e., Europe–America] or East [i.e., Asia–Africa])
and the extent of their own and their father’s formal
education and attainment of an academic degree. In addition,
participants were asked about the age (rounded to the nearest
half year) at the onset of menstruation, present smoking status
(current smoker, past smoker, or never a smoker), age at onset of
habitual smoking (defined as >1 cigarette/week), age at first OC use, and current status of OC use. The study and data
collection were approved by the IDF Ethics Committee.
Statistical analysis
Main outcomes (age at menarche, age at first cigarette, and
age at first use of OC) were compared between birth cohorts using
one-way analysis of variance after grouping the study population
into five cohorts based on birth year (prior to 1970,
1970 to 1972, 1973 to 1975, 1976 to 1978, and after 1978).
To determine changes over time in age of menarche, data were
initially analyzed by employing a simple linear regression model
with age at menarche as the dependent variable and year of birth
as the sole covariate. To investigate the possible influence of
paternal sociodemographic background on the temporal trend in age
at menarche, similar linear regression models were fitted, one
for each category of the origin (West and East), age of initial
smoking and smoking status at recruitment (current smoker, past smoker, and
never a smoker), and father’s years of education (categorized into
<12 years, 12 years, and >12 years of education).
To assess differences
in age at menarche by birth cohort, a term for year of birth and
background variables were included in a stepwise multivariate
model, using age at menarche as the dependent variable. Analyses were
carried out using standard statistical software (SPSS 11.0,
Chicago, Ill).
Back to top
Results
Data were available for 11,392 women, with a mean (± SD)
age of 18.5 (± 0.37) years and a median of 18.47 years at
recruitment. A trend of increasing education over time was
recorded for subjects’ fathers. Approximately 43.5% of the
study participants were of western origin, with little
variation over time (Table 1).
Reported mean age (± SD) at menarche showed a monotonic
trend of decrease over time, from 13.41 (± 1.30) years for
women born before 1970 (the earliest birth cohorts in the study
population) to 13.03 (± 1.28) years for those born after
1978 (the latest birth cohorts in the study population) (P
< .001) (Table 2). The
proportion of subjects reporting menarche at age 11 or earlier was 3.3% for
women born before 1970, 4.0% for women born from 1970 to 1972, 4.9% for women
born from 1973 to 1975, 5.2% for women born from 1976 to 1978, and 6.3% for
women born after 1978. Women born after 1978 were twice as likely as those born
before 1970 to have had an early menarche (odds ratio 2.0; 95% CI, 1.41–2.82).
The study data showed a significant increase over time in the
proportion of recruits reporting having ever smoked (from 23.55%
in the earliest birth cohort to 32.86% in the latest [P
< .001]) and in those reporting having ever used OC (from
25.49% in the earliest birth cohort to 31.24% in the latest
[P < .001]) (Figure). A significant downward trend was
observed in mean age at first use of OC with sequential birth cohorts, from
17.49 (± 0.81) years in the earliest cohort to 17.14 (± 1.05) years in the latest cohort (P < .001).
A similar trend was present for age at first cigarette, with the
mean age decreasing from 16.68 (± 1.30) years to 15.85 (±
1.36) years (P < .001).
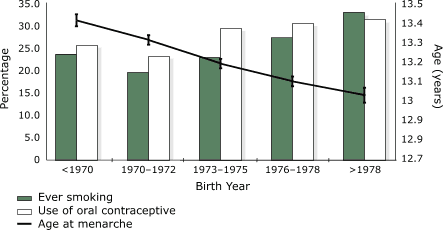
Figure.
Trend for age at menarche (P < .001) and proportion of
recruits having ever smoked (P < .001) or used oral contraceptives (P
< .001) among female Israeli army recruits (1986–2000), by birth year. The left axis refers
to prevalence (%) of positive smoking history (green columns) and oral
contraceptive use (white columns). The right axis refers to mean reported age in
years at menarche. [A tabular version of this graph
is available.]
Regression modeling showed a negative association between the
recruits’ year of birth and their age at menarche,
indicating a downward temporal trend of 4.2 months per decade
(95% CI, 3.6–5.0) in age at menarche. Comparison of
regression coefficients between birth year and reported age at
menarche shows similar results for women of western and eastern
origins and for all categories of paternal education (Table 3). A
tendency toward a stronger downward temporal trend was recorded
among past smokers; however, this trend did not reach statistical
significance. Year of birth was the sole factor that maintained a
significant regression coefficient with age at menarche in a
multivariate linear regression model that also included
subject’s origin, father’s level of education, and
smoking status.
Back to top
Discussion
This population-based study of young Israeli women
demonstrates a trend over time of decreasing age at menarche, as
indicated by younger age at menarche among girls born between
1965 and 1981. These same birth cohorts were characterized by
decreasing age at first cigarette and at first use of OC and by
an increasing proportion of adolescent smoking and OC use.
Age at first menstruation has shown a decreasing secular trend
in many populations (12-14). A recently published study of a nationally representative sample of U.S. girls shows that for 80%
of girls in the United States, menarche occurs between 11.00 and
13.75 years of age, with a median of 12.43 years (2). Although
our study data show a relatively older average age at menarche in
comparison with the United States, a steeper trend of reduction
over time was recorded. Although data from the United States
suggest an overall drop of approximately 2.5 months during the
period between the late 1960s and the early 1990s
(15), our data indicate a decrease of 4.2 months per decade.
Scandinavian studies have shown a trend toward younger age at
menarche during the second half of the 20th century (16). A
previous study undertaken in Israel in 1977 among a sample of 285
Jewish girls in grades three through eight (birth years 1969 and
earlier) found a mean age of 13.29 (± 0.45) years at menarche
(9). This mean was nearly identical to that found in the current
study among girls of comparable birth cohorts.
The ecologic association between age at menarche and age of
first cigarette may have several possible explanations. Girls who
are relatively physically mature for their age may associate with
older adolescents and imitate their behavior (17). Menarche at
younger age may present a serious psychological stressor,
resulting in early smoking in addition to other unhealthy behaviors. Several studies have suggested that hormonal changes
accompanying menarche may directly contribute to changes in
behavior (18,19).
This population-based study has several strengths, such as
systematic sampling, large sample size, and standardized data
collection methods throughout the entire study period. Because
young women with severe disease or disability are exempt from
military service, study data are not affected by potential
secular changes in the epidemiology of such diseases in the
general population. Two limitations must be noted, however. Since
ultra-Orthodox Jewish and Arab adolescents are exempt from
military service, no conclusions pertaining to these minority
groups can be drawn from the present study. Additionally, data
collection for this study was based on recall of age at menarche
at recruitment rather than recorded prospectively at time of
onset. However, because menarche is an easily recognizable event
that is well remembered by most women, any reporting errors are
expected to be both small and nondifferential because the study
participants were all of the same age at recruitment and data
were obtained only a few years after menstruation commenced.
It is generally thought that improvement in socioeconomic
conditions and overall health are the main contributing factors
to the trend toward earlier sexual maturation (20). Nonetheless,
the mechanisms through which these changes occur are still
unknown. Several hypotheses have been discussed in the
literature, such as the “critical weight hypothesis” (21) and the leptin hypothesis (22). Other investigators have studied the role
of estrogen-like substances in the environment on the timing of
puberty (23). However, stabilization in the age of puberty onset
over the last two decades, which has been observed in several
countries (24,25), and studies finding that girls belonging to
lower social strata showed a temporal trend toward reduction in
the age at menarche (9,26,27) raise questions about the
causality of these factors.
A recent cohort study of 17,032 women aged 25 to 39 who had
used OC showed that death from all causes was more than twice as
high in smokers (≥15 cigarettes per day) than in
nonsmokers and that harmful effects were already apparent in
women aged 35 to 44 (26). The age at which smoking begins has
been shown to influence the total number of years of smoking
(28), the number of cigarettes smoked per day in adulthood, and
the likelihood of quitting (29), all of which affect the risk for
developing smoking-attributable disease and disability (30).
The ongoing significant growth in the proportion of adolescent
girls who smoke at an increasingly younger age, combined with the
rise in OC use and increasingly younger ages at menarche and
initial OC use, suggest a rise not only in the prevalence of
exposure to these risk factors but also in the duration of
exposure. This trend implies a serious public health problem,
which may translate into increased future morbidity and
mortality. Given the epidemiology of smoking initiation, more
public health policy and programmatic attention should be aimed
at early adolescence in order to prevent or delay adolescent
cigarette smoking. This approach strongly supports the Centers
for Disease Control and Prevention’s “Guidelines for
School Health Programs to Prevent Tobacco Use and Addiction,”
which call for tobacco-use prevention education in kindergarten
through 12th grades (30). Health policy authorities should
further investigate changes in health behavior among adolescents
over time to evaluate the effectiveness of such intervention in
the future.
Back to top
Author Information
Corresponding Author: Gabriel Chodick, PhD, MHA, Israel Defense Force Medical
Corps, Army Health Branch, Department of
Epidemiology & Preventive Medicine, Sackler Faculty of Medicine, Tel-Aviv University, PO Box 39040, Ramat Aviv, Tel Aviv
69978, Israel. Telephone: 972-3-6409040. E-mail: hodik_g@mac.org.il.
Author Affiliations: Michael Huerta, MD, MPH, Ran D. Balicer, MD, Nadav Davidovitch,
MD, MPH, Itamar Grotto, MD, MPH, Israel
Defense Force Medical Corps, Army Health Branch, Department of
Epidemiology & Preventive Medicine, Sackler Faculty of
Medicine, Tel Aviv University, Tel Aviv, Israel.
Back to top
References
- Dewhurst J. Female Puberty and its Abnormalities. London:
Churchill Livingstone; 1984.
- Chumlea WC, Schubert CM, Roche AF,
Kulin HE, Lee PA, Himes JH, et al.
Age at menarche and racial comparisons in US girls.
Pediatrics 2003;111:110-3.
- Wyshak G, Frisch RE.
Evidence for a secular trend in age of
menarche. N Engl J Med 1982;306:1033-5.
- Harlan WR, Harlan EA, Grillo GP.
Secondary sex characteristics
of girls 12 to 17 years of age: the U.S. Health Examination
Survey. J Pediatr 1980 Jun;96(6):1074-8.
- Kimm SY, Barton BA, Obarzanek E,
McMahon RP, Sabry ZI, Waclawiw MA, et al.
Racial divergence in adiposity during adolescence:
the NHLBI
Growth and Health Study. Pediatrics 2001;107:E34.
- Clavel-Chapelon F; E3N-EPIC Group.
Differential effects of reproductive factors on the risk of pre- and postmenopausal
breast cancer. Results from a large cohort of French women. Br J
Cancer 2002;4:723-7.
- Burkman RT.
Cardiovascular issues with oral contraceptives:
evidenced-based medicine. Int J Fertil Womens Med
2000;45:166-74.
- Vessey M, Painter R, Yeates D.
Mortality in relation to oral
contraceptive use and cigarette smoking. Lancet
2003;362:185-91.
- Belmaker E.
Sexual maturation of Jerusalem schoolgirls and
its association with socio-economic factors and ethnic group. Ann
Hum Biol 1982;9:321-8.
- Kark JD, Laor A.
Cigarette smoking and educational level among
young Israelis upon release from military service in 1988--a
public health challenge. Isr J Med Sci 1992;28:33-7.
- Grotto I, Huerta M, Kark JD,
Shpilberg O, Meyerovitch J.
Relation of parental history of coronary heart disease to obesity
in young adults. Int J Obes Relat Metab Disord
2003;27(3):362-8.
- Harper J, Collins JK.
The secular trend in the age of menarche in
Australian schoolgirls. Aust Paediatr J 1972;8:44-8.
- Maresh M.
A forty-five year investigation for secular
changes in physical maturation. Am J Phys Anthropol
1972;36:103-10.
- Tanner JM.
Trend towards earlier menarche in London, Olso,
Copenhagen, the Netherlands and Hungary. Nature
1973;243:95-6.
- Anderson SE, Dallal GE, Must A.
Relative weight and race
influence average age at menarche: results from two nationally
representative surveys of US girls studied 25 years apart.
Pediatrics 2003;111:844-50.
- Helm P, Helm S.
Decrease in menarcheal age from 1966 to 1983
in Denmark. Acta Obstet Gynecol Scand 1984;63:633-5.
- Brooks-Gunn J.
Antecedents and consequences of variations
in girls' maturational timing. J Adolesc Health Care
1988;9:365-73.
- Susman EJ, Nottelmann ED,
Inoff-Germain G, Dorn LD, Chrousos GP.
Hormonal influences on aspects of psychological development
during adolescence. J Adolesc Health Care 1987;8:492-504.
- Warren MP, Brooks-Gunn J.
Mood and behavior at adolescence: evidence
for hormonal factors. J Clin Endocrinol Metab 1989;69:77-83.
- Hauspie RC, Vercauteren M, Susanne
C.
Secular changes in growth and
maturation: an update. Acta Paediatr Suppl 1997;423:20-7.
- Frisch RE, Revelle R.
Height and weight at menarche and a
hypothesis of critical body weights and adolescent events.
Science 1970;169:397-9.
- Clayton PT, Trueman JA.
Leptin and puberty. Arch Dis Child
2000;83:1-4.
- Mazur W.
Phytoestrogen content in foods. Baillieres Clin
Endocrinol Metab 1998;12:729-42.
- Mul D, Fredriks AM, van Buuren S,
Oostdijk W, Verloove-Vanhorick SP, Wit JM.
Pubertal development in The
Netherlands 1965-1997. Pediatr Res 2001;50:479-86.
- Liestol K, Rosenberg M.
Height, weight and menarcheal age
of schoolgirls in Oslo—an update. Ann Hum Biol
1995;22:199-205.
- Prebeg Z.
Changes in growth patterns in Zagreb school
children related to socioeconomic background over the period
1973-1991. Ann Hum Biol 1998;25:425-39.
- Junqueira Do Lago M, Faerstein E, De Souza Lopes C,
Werneck GL; Pro-Saude Study (Rio de Janeiro, Brazil).
Family
socio-economic background modified secular trends in age at
menarche: evidence from the Pro-Saude Study (Rio de Janeiro,
Brazil). Ann Hum Biol 2003;30:347-52.
- Escobedo LG, Marcus SE, Holtzman D, Giovino GA.
Sports
participation, age at smoking initiation, and the risk of smoking
among US high school students. JAMA 1993 17;269:1391-5.
- Breslau N, Fenn N, Peterson EL.
Early smoking initiation
and nicotine dependence in a cohort of young adults. Drug Alcohol
Depend 1993;33:129-37.
- Centers for Disease Control and Prevention.
Guidelines for
school health programs to prevent tobacco use and addiction. MMWR Recomm
Rep. 1994 Feb 25;43(RR-2):1-18.
Back to top |
|
