Volume 16, Number 2—February 2010
Research
Domestic Animals and Epidemiology of Visceral Leishmaniasis, Nepal
Abstract
On the Indian subcontinent, visceral leishmaniasis (VL) is considered an anthroponosis. To determine possible reasons for its persistence during interepidemic periods, we mapped Leishmania infections among healthy persons and animals in an area of active VL transmission in Nepal. During 4 months (September 2007–February 2008), blood was collected from persons, goats, cows, and buffaloes in 1 village. Leishmania infections were determined by using PCR. We found infections among persons (6.1%), cows (5%), buffaloes (4%), and goats (16%). Data were georeferenced and entered into a geographic information system. The bivariate K-function results indicated spatial clustering of Leishmania spp.–positive persons and domestic animals. Classification tree analysis determined that among several possible risk factors for Leishmania infection among persons, proximity of Leishmania spp.–positive goats ranked first. Although our data do not necessarily mean that goats constitute a reservoir host of L. donovani, these observations indicate the need for further investigation of goats’ possible role in VL transmission.
Visceral leishmaniasis (VL), also known as kala-azar, is a fatal vector-borne parasitic disease. Worldwide incidence is 500,000 cases per year; ≈90% of cases occur in India, Nepal, Bangladesh, Sudan, and Brazil (1). On the Indian subcontinent, the number of officially reported cases, although only a fraction of the true incidence (2), has increased during the past 5–6 years, and the disease is spreading to new areas (3). A kala-azar elimination program was recently launched by the governments of Bangladesh, India, and Nepal, with the support of the World Health Organization; the goal is to reduce the annual incidence in VL-in endemic regions to <1 case per 10,000 persons by 2015 (4). The program essentially relies on early diagnosis and treatment of persons and on vector control (5). This strategy is based on the assumption that Leishmania donovani, the etiologic agent of VL, is transmitted from person to person (anthroponotic VL).
The possible role of domestic animals in anthroponotic VL has been studied in Bangladesh (6), but no clear conclusions have been drawn with regard to animals as risk factors or reservoir hosts. In contrast, the proximity to a VL-infected person is a major risk factor for VL (6). Thus, persons are still considered the only reservoir host for L. donovani on the Indian subcontinent. However, the reasons for persistence during interepidemic periods are debated, and dermal leishmaniasis after kala-azar has been incriminated (7). Correct identification of the Leishmania reservoir host is crucial for the design of control programs. Molecular tools offer new opportunities to better document and reassess transmission patterns. To explore the potential role of domestic animals in transmission, we performed an extensive study in an area of active VL transmission in Nepal, mapping Leishmania infections among healthy persons and domestic animals.
Study Site
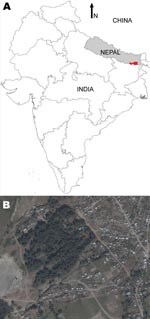
The study was conducted as part of the KALANET project, a community trial of insecticide-treated bed nets (www.kalanetproject.org) in the Terai region of eastern Nepal. In Nepal, each village is divided into several wards. For the KALANET project, 10 wards with active VL transmission were selected. Dharan-17 was 1 such ward; it is a periurban ward in the Dharan municipality, located in the foothills of the Mahabharata hills and along the bank of Sardu River. Dharan-17 covers ≈0.3 km2 (Figure 1) and has 515 inhabitants living in 105 households (Figure 2). A demographic survey conducted in July 2006 showed that 77% of households had at least 1 domestic animal (i.e., cow, goat, dog). Most cows, buffaloes, and goats were kept <10 m from the households at night, although a few goats were kept inside the house. VL was only recently reported in this periurban area; Dharan-17 has an average VL incidence rate of 1.61% per year (for 2004–2006). Furthermore, during a previous study conducted in 2006, we documented a higher rate of VL positivity by PCR among the healthy persons in Dharan-17 compared with those in the 9 other wards in the KALANET trial, possibly suggesting a high transmission rate (8). For a control area, we selected Dhankura-3 in Patlekhola. This ward is ≈60 km from Dharan-17, in a hilly area where no VL cases have yet been reported.
Ethical Aspects
Ethical clearance for the KALANET project was obtained from the Ethical Committee of the B.P. Koirala Institute of Health Sciences (BPKIHS), Dharan, Nepal, and the corresponding bodies at the Institute of Tropical Medicine, Antwerp, Belgium, and the London School of Hygiene and Tropical Medicine, London, UK. A community meeting informed local leaders and village residents about the study purpose; informed consent was obtained from all animal owners before their animals were included in the study. International animal experimentation guidelines were followed. Persons provided written consent before enrollment and providing blood samples, per the human experimentation guidelines approved by BPKIHS and the corresponding body at the Institute of Tropical Medicine, Antwerp. For religious reasons, 10.67% of persons did not provide written consent to donate their blood samples.
Sample Collection
All animal surveys were conducted by experienced veterinarians. In Dharan-17, a house-to-house survey was first conducted in September 2007, among 105 households, to collect information on the number and types of animals present in the ward; only information about bovines (cows and buffaloes) and goats was collected in this first survey. Later, 2 sampling surveys were conducted in this ward. In October 2007, survey I sampled 144 goats, 24 buffaloes, and 20 cows from the 37 households that had >1 bovine or goat. In February 2008, survey II focused on 6 households in which Leishmania spp.–positive animals had been identified during survey I. Although the owners claimed that the goats in surveys I and II were the same goats, we could not confirm this. In February 2008, samples were collected from 25 goats, 17 buffaloes, and 21 cows in the control area. In addition to animal samples, we also collected 278 blood samples from persons, all >5 years of age, who lived in Dharan-17 at the time of the survey and provided consent. The samples (1 mL) were collected by venipuncture from animals and persons into tubes containing molecular biology grade Na2EDTA (240 μg/mL of blood; Sigma-Aldrich, Bornem, Belgium). All tubes were immediately stored in a chilled ice box and transferred on the same day to the laboratory at BPKIHS, where 180 μL of each sample was transferred to a tube containing 180 μL of AS1 buffer (catalog no. 1006243; QIAGEN, Venlo, the Netherlands), mixed well, and stored at room temperature.
DNA Extraction and PCR Amplification
All blood samples stored in AS1 buffer were used to extract the DNA within 1 month. The QIAamp DNA Mini Kit (catalog no. 56301; QIAGEN) was used to extract DNA at BPKIHS, following manufacturer’s instructions. All DNA samples were sent at ambient temperature to the Institute of Tropical Medicine, Antwerp, where they were analyzed by PCR specific for small ribosomal genes of Leishmania spp. as described elsewhere (9). To confirm that the amplified DNA corresponded to Leishmania spp., amplicons from a set of positive samples were sequenced. The sequences were compared with those of Leishmania spp. and other trypanosomatids from GenBank.
Spatial Clustering of Leishmania spp.–positive Households
We used the results from survey I to assess the clustering of the Leishmania spp.–positive samples in Dharan-17. Each household, previously georeferenced by a geographic positioning system and mapped by using ArcGIS 9.2 (ESRI, Redlands, CA, USA), was identified as Leishmania spp.–positive or –negative for animal and human samples. Analyses considered all animals (goats, cows, and buffaloes) together. The bivariate K-function was used to determine whether households with Leishmania spp.–positive persons were spatially clustered around households with Leishmania spp.–positive domestic animals in Dharan-17. The following equation was used (10): K(d) = expected no. events B within distance d of arbitrary event A / overall density of events B. For easier interpretation of the results, the bivariate K-function was transformed in an L-function as follows (11):
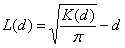
Positive L(d) results would suggest that human and animal Leishmania spp.– positive households are spatially associated. Further technical details on the bivariate K-function are available in the Technical Appendix(10–12).
Classification Trees
Classification trees (CTs) and regression trees (Salford Systems, San Diego, CA, USA) can be used in classification and regression problems (13). We used CTs to analyze risk factors and identify interactions for Leishmania spp.–positive households. This nonlinear, assumption-free, and algorithm-based method splits data variance across nested nodes (with increased importance toward the tree base). Its algorithm automatically eliminates variables without explanatory power. Sensitivity (% true positives) and specificity (% true negatives) were computed. A 10-fold cross-validation (leaving 10% of the data at a time for the whole dataset to compute percentage of misclassifications) was used. Node impurity was measured by using the Gini index, and the minimal size of the base and terminal nodes were set to 10 and 1 cases, respectively. The method is explained in more detail elsewhere (Technical Appendix) (14–17). The factors included in the CT analysis were 1) household (i.e., type of house, head of household occupation/education) and biologic (i.e., density of domestic animals around households, collected in a house-to-house survey in July 2006 and estimated by using a kernel density function [ArcGIS, ESRI]) (6) and 2) results of PCR analyses (Table 1).
Animal Sample PCR Results
Survey I found 188 domestic animals (goats, buffaloes, and cows) in only 37 of the 105 households of Dharan-17. The overall rate of Leishmania positivity was 13.3% (25/188); goats accounted for 16% (23/144), cows for 5% (1/20), and buffaloes for 4% (1/24).
The Leishmania spp.–positive animals were encountered in the 15 households shown in Figure 2. During survey II, only goats were sampled from 6 of these households; 2 goats (from different households) of 24 (8.34%) were Leishmania spp. positive. All sequenced amplicons confirmed the presence of a Leishmania-specific sequence. All 63 samples from animals in the control area were negative.
Human Sample PCR Results and Spatial Clustering
In Dharan-17, of the 278 persons sampled, 17 (6.1%) were Leishmania spp. positive, 14 were healthy with no history of kala-azar, and none was a household contact of a VL case-patient. Of the 17 Leishmania spp.–positive persons, 2 had had VL and had been successfully treated. We could not determine the history for 1 person because he had moved out of the ward. The 17 Leishmania spp.–positive persons were from 16 households. The Kuldorff spatial scan statistic (18) was used to assess whether Leishmania spp.–positive persons were clustered in Dharan-17, but no significant clusters were detected. Analogous results were obtained for Leishmania spp.–positive animals (results not shown). However, when we superimposed households and Leishmania spp.–positive persons or animals (Figure 2), it visually appeared that 1) in 8 sites of the ward, Leishmania spp.–positive persons were localized in households near where Leishmania spp.–positive animals were kept (distance between the households <30 m; further called clusters), and that 2) in 1 site only Leishmania spp.–positive persons were found (Table 2). Some clusters constituted hot spots (had several cases of infection): clusters 2 (9 animals and 2 persons) and 8 (6 animals and 2 persons). The clusters represented in Figure 2 and detailed in Table 2 were determined visually; no statistical methods were applied. The bivariate K-function results show that households with Leishmania spp.–positive persons were clustered around households with Leishmania spp.–positive animals; L(d) is positive from 0 to 100 m. However, the spatial association between them is only significant from 0 to 5 m (Technical Appendix).
Classification Tree Analysis
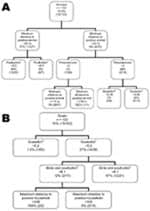
The bivariate K-function analyzes only the relationship between Leishmania spp.–positive households for persons and domestic animals in Dharan. The results of this function have no meaning other than a spatial grouping of households with positive animals and persons. In a second stage, we used a CT model to analyze risk factors for Leishmania spp.–positive households. First we analyzed households in which Leishmania spp.–positive persons had been encountered. These results showed that the minimum distance to a household with a Leishmania spp.–positive animal (any species) was the variable with the highest discriminatory power. It appears first in the tree (Figure 3, panel A) and gets a relative importance score of 100% (data not shown). Discriminating distance was 22.8 m: households <22.8 m from a household with Leishmania spp.–positive animals showed a 37% probability of hosting Leishmania spp.–positive persons versus 7.9% if they were >22.8 m from a household with Leishmania spp.–positive animals. The next variables appearing on the tree were the density in poultry (higher risk for Leishmania spp.–positive persons if density <8.55/km2), the number of persons per room (higher risk if >2.88 persons/room), and density of goats (higher risk if >0.05 goats/ km2). A second CT analysis was conducted for households in which Leishmania spp.–positive animals had been encountered. The generated tree differed from the previous one in that the first discriminating variable was the density of goats per km2; risk for Leishmania spp. positivity associated with a density >5.2 (36.8% vs. 1.5%; Figure 3, panel B) was higher, and a relative importance score was 100% (data not shown). The next variables appearing on the tree were the density of birds and poultry per km2 (higher risk for Leishmania spp.–positive goats if >6.14) and the maximum distance to a household with a Leishmania spp.–positive person (higher risk if <334.6 m). All these variables also appeared with highest predictor ranking scores (data not shown).
For the tree in Figure 3, panel A, the sensitivity and specificity of the tree were 100% and 80.5%, respectively. For the tree in Figure 3, panel B, sensitivity and specificity were 93.3% and 89.8%, respectively.
The minimum distance to a positive animal split in Figure 3, panel A, is difficult to interpret. It involves 1 positive result and is under a branch that was determined to be predominantly negative as a result of the first split when using the same variable at >23 m. This split should therefore be interpreted with caution. The same applies for the split of maximum distance to positive household <335 m (Figure 3, panel B), which is difficult to interpret if the study area is only 300 m2 and may be the result of the irregular shape of the study area.
We found Leishmania DNA in domestic animals (cows, buffaloes, and goats) from Dharan-17, mostly in goats (16%), although no goats or other animals in the control area were Leishmania spp. positive. As DNA persists in the body for only a short time (24 h) (19), PCR positivity is a good indicator of current (or recent) infection. Considering the time between the Leishmania spp. peak transmission season in Nepal (estimated in April–May) (20) and our first survey (October 2007), our results thus indicate that goats can be infected with L. donovani and that this infection persists for at least several months. Contact with Leishmania organisms, as shown by serologic findings, has already been reported for goats and other domestic animals in Sudan (21).
The decrease in Leishmania spp. positivity between the 2 surveys (from 16% in October 2007 to 8.34% in February 2008) could be explained by several reasons: 1) sampling bias, 2) effective decrease of parasitemia over a certain period because of immunologic control of infection, or 3) disappearance of positive animals from the ward. A follow-up study of Leishmania spp.–infected goats (up to at least 12 months) combined with an adequate tracking system are needed to determine whether parasites are still in the blood of the animals during the next transmission peak.
Comparison of the results from animals with those from healthy human volunteers from Dharan-17 provided additional interpretation of the animal results. Leishmania spp. positivity was found to be ≈3× lower among persons than among animals (6.1% vs. 16%, respectively). These data are consistent with data on the feeding behavior of Phlebotomus argentipes blood-sucking flies, reported previously (22). This species seems to breed essentially in cattle sheds (23) and are 5× more attracted to cattle than to persons (24,25) and feed more on animals (62.80%) than on persons (24.92%), according to a study in India (22).
Information about the association of PCR results for persons and domestic animals can be used to investigate the role of domestic animals in L. donovani transmission. Visual inspection of the data suggested that most of the Leishmania spp.–positive persons were living near Leishmania spp.–positive goats. This observation was confirmed by bivariate K-function results and CT analysis. Distance of clustering between Leishmania spp.–positive persons and Leishmania spp.–positive domestic animals varied slightly according to the method (K-function up to 5 m; CT analysis <22.8 m) and was less than the flight range of P. argentipes flies. Differences could be explained by the inclusion of Leishmania spp.–negative households in the CT analysis. The 2 types of CT analyses pointed more to the role of biologic factors than to household factors like education, bed-net use, or type of house. Additional studies should explore the presence of poultry as a risk factor, as has been reported for urban VL in Brazil (26).
Even if our results indicate that goats might be involved in the dynamics of VL, they do not necessarily mean that these animals constitute a reservoir host for L. donovani. Criteria for the definition of Leishmania reservoir hosts were recently reviewed (27) and include sandfly foraging behavior and feeding preferences and the dynamics of infections in assumed reservoir hosts; a key question is the clearance times (chronicity) of infections. Whether the phenomenon observed here can be extrapolated to other VL-endemic foci should also be explored. Dharan-17 is a new emerging focus, and in the absence of immunity, human and animal populations could be more sensitive to Leishmania infections. Our observations warrant further investigation and a close monitoring of goats and other peridomiciliary animals like rodents and birds. If the role of these animals in the transmission cycle is confirmed, the potential implications could affect VL control programs in the region.
Mr Bhattarai is a PhD student in the laboratory of molecular parasitology of the Institute of Tropical Medicine, Antwerp, Belgium. His research interests are molecular diagnosis and epidemiology of leishmaniasis.
Acknowledgments
We thank Ganesh Prasad Regmi for invaluable help collecting blood samples from animals; the field workers Tikaram Khatri, Ram Bahadur Lama, and Thule for carefully handling all domestic animals during sampling; Tika-Ram Koirala for vehicle support; and Surendra Uranw for arranging a community meeting and supervising blood collection from human participants. Finally, the study would not be possible without the help of the Dharan-17 villagers, who showed a keen interest in including their animals and their own blood samples in our study.
This study was supported by the European Union–funded INCO-DEV KALANET project (EU contract no 015374).
References
- Desjeux P. Leishmaniasis. Public health aspects and control. Clin Dermatol. 1996;14:417–23. DOIPubMedGoogle Scholar
- Singh SP, Reddy DC, Rai M, Sundar S. Serious underreporting of visceral leishmaniasis through passive case reporting in Bihar, India. Trop Med Int Health. 2006;11:899–905. DOIPubMedGoogle Scholar
- Joshi AB, Banjara MR, Pokhrel S, Jimba M, Singhasivanon P, Ashford RW. Elimination of visceral leishmaniasis in Nepal: pipe-dreams and possibilities. [KUMJ]. Kathmandu Univ Med J. 2006;4:488–96.
- World Health Organization. Regional strategic framework for elimination of kala-azar from the South-East Asia region (2005–2015). New Delhi (India): The Organization; 2005 [cited 2009 Dec 11]. http://www.searo.who.int/LinkFiles/Kala_azar_VBC-85_Rev_1.pdf
- Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, Desjeux P, Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis. 2002;2:494–501. DOIPubMedGoogle Scholar
- Bern C, Hightower AW, Chowdhury R, Ali M, Amann J, Wagatsuma Y, Risk factors for kala-azar in Bangladesh. Emerg Infect Dis. 2005;11:655–62.PubMedGoogle Scholar
- Addy M, Nandy A. Ten years of kala-azar in west Bengal, Part I. Did post–kala-azar dermal leishmaniasis initiate the outbreak in 24-Parganas? Bull World Health Organ. 1992;70:341–6.PubMedGoogle Scholar
- Bhattarai NR, Van der Auwera G, Khanal B, De Doncker S, Rijal S, Das ML, PCR and direct agglutination as Leishmania infection markers among healthy Nepalese subjects living in areas endemic for kala-azar. Trop Med Int Health. 2009;14:404–11. DOIPubMedGoogle Scholar
- Deborggraeve S, Boelaert M, Rijal S, De Doncker S, Dujardin JC, Herdewijn P, Diagnostic accuracy of new Leishmania PCR for clinical visceral leishmaniasis in Nepal and its role in diagnosis of disease. Trop Med Int Health. 2008;13:1378–83. DOIPubMedGoogle Scholar
- Lotwick HW, Silverman BW. Methods for analysing spatial processes of several types of points. [Ser A]. J R Stat Soc [Ser A]. 1982;B44:406–13.
- Bailey TC, Gatrell AC. Interactive spatial data analysis. New York: John Wiley & Sons; 1995. p. 120–2.
- Ripley BD. The second-order analysis of stationary point process. J Appl Probab. 1976;13:255–66. DOIGoogle Scholar
- Olden JD, Lawler JJ, Poff NL. Machine learning methods without tears: a primer for ecologists. Q Rev Biol. 2008;83:171–93. DOIPubMedGoogle Scholar
- Cook EF, Goldman L. Empiric comparison of multivariate analytic techniques: advantages and disadvantages of recursive partitioning analysis. J Chronic Dis. 1984;37:721–31. DOIPubMedGoogle Scholar
- Thang ND, Erhart A, Speybroeck N. Hung le X, Thuan le K, Hung TK. Malaria in central Vietnam: analysis of risk factors by multivariate analysis and classification tree models. Malar J. 2008;7:28. DOIPubMedGoogle Scholar
- Saegerman C, Speybroeck N, Roels S, Vanopdenbosch E, Thiry E, Berkvens D. Decision support tools in clinical diagnosis in cows with suspected bovine spongiform encephalopathy. J Clin Microbiol. 2004;42:172–8. DOIPubMedGoogle Scholar
- Speybroeck N, Berkvens D, Mfoukou-Ntsakala A, Aerts M, Hens N, Van Huylenbroeck G, Classification trees versus multinomial models in the analysis of urban farming systems in central Africa. Agric Syst. 2004;80:133–49. DOIGoogle Scholar
- Kulldorff MA. Spatial scan statistic. Communications in statistics—theory and methods. 1997;26:1481–96.
- Prina E, Roux E, Mattei D, Milon G. Leishmania DNA is rapidly degraded following parasite death: an analysis by microscopy and real-time PCR. Microbes Infect. 2007;9:1307–15. DOIPubMedGoogle Scholar
- Bhandari GP. Kala-azar control programme in Nepal: a programme review from 1994–2006 [dissertation: IMTA/MCM-MDC; 173]. Antwerp (Belgium): Prince Leopold Institute of Tropical Medicine; 2008.
- Dereure J, El-Safi SH, Bucheton B, Boni M, Kheir MM, Davoust B, Visceral leishmaniasis in eastern Sudan: parasite identification in humans and dogs; host–parasite relationships. Microbes Infect. 2003;5:1103–8. DOIPubMedGoogle Scholar
- Palit A, Bhattacharya SK, Kundu SN. Host preference of Phlebotomus argentipes and Phlebotomus papatasi in different biotopes of West Bengal, India. Int J Environ Health Res. 2005;15:449–54. DOIPubMedGoogle Scholar
- Singh R, Lal S, Saxena VK. Breeding ecology of visceral leishmaniasis vector sandfly in Bihar state of India. Acta Trop. 2008;107:117–20. DOIPubMedGoogle Scholar
- Dinesh DS, Ranjan A, Palit A, Kishore K, Kar SK. Seasonal and nocturnal landing/biting behaviour of Phlebotomus argentipes (Diptera: Psychodidae). Ann Trop Med Parasitol. 2001;95:197–202. DOIPubMedGoogle Scholar
- Lioyd RB, Naiper LE. The blood meal of sand-flies investigated by means of precipitin antisera. Indian J Med Res. 1930;18:347–59.
- Alexander B, de Carvalho RL, McCallum H, Pereira MH. Role of the domestic chicken (Gallus gallus) in the epidemiology of urban visceral leishmaniasis in Brazil. Emerg Infect Dis. 2002;8:1480–5.PubMedGoogle Scholar
- Chaves LF, Hernandez MJ, Dobson AP, Pascual M. Sources and sinks: revisiting the criteria for identifying reservoirs for American cutaneous leishmaniasis. Trends Parasitol. 2007;23:311–6. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 16, Number 2—February 2010
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Jean-Claude Dujardin, Prins Leopold Institute, Nationalestraat, 155 Antwerp 2000, Belgium
Top